Published: 22 March 2017
Revised: 29 August 2023
Safety Information
Trans-Tasman Early Warning System — Alert Communication
Consumer Level Recall – EpiPen® (Adrenaline) 300 micrograms (mcg) Auto-injector
22 March 2017
Products Affected
Information for consumers and caregivers
Information for healthcare professionals
What action is Medsafe taking?
How to report adverse events
Description
This recall is being undertaken as a precautionary measure due to the potential that these devices may contain a defective part that may result in the device failing to activate or requiring increased force to activate.
Products Affected
| The product is: | EpiPen® (Adrenaline) 300 micrograms (mcg) Auto-injector |
|---|---|
| Batch Numbers: | 5FA665 and 5FA6652 |
Information for consumers and caregivers
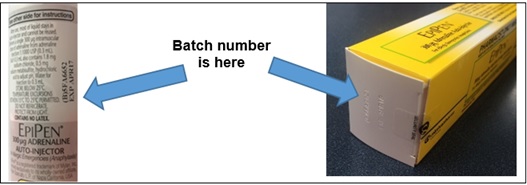
- Please check the batch number on your EpiPen® Auto-Injector. The batch number can be found on the end of the carton or on the label of the pen.
- If you are in possession of an EpiPen® Auto-Injector with the batch number 5FA665 or 5FA6652, please return it to your pharmacy and you will be given a replacement EpiPen® Auto-Injector from a different batch FREE OF CHARGE.
- If you cannot get to your pharmacy immediately or your pharmacy needs to order a replacement for you, please retain your EpiPen® Auto-Injector until the replacement can be provided and use it if required as the risk of the device failing to activate is very low.
- If you are in possession of an EpiPen® Auto-Injector with a different batch number or a green EpiPen® Jr 150mcg, your product is NOT affected by this recall and can be kept for use if needed.
Information for healthcare professionals
- This recall only applies to EpiPen® Auto-Injector from batches 5FA665 and 5FA6652.
- Replacement stock from an unaffected batch is available. Please place an order with Mylan for either direct supply or by nominating your preferred wholesaler.
- Mylan is instructing consumers to return their EpiPen® to their pharmacy for a free replacement, if they possess stock from the affected batch.
- If a consumer presents with an EpiPen® from the affected batches, replace the device at no cost with an EpiPen® from a non-affected batch.
- In the event that you do NOT have stock from unaffected batches when a patient presents, please advise the patient to retain their current EpiPen® from the affected batch until a replacement can be provided as the risk of the device failing to activate is very low.
What action is Medsafe taking?
Monitoring the consumer level recall of EpiPen® 300mcg Auto-Injector with the batch numbers 5FA665, and 5FA6652, and monitoring any adverse events that may be reported.
How to report adverse events
| Online |
Submit a CARM report Prescribers can also submit a report using the online reporting tool available in patient management software. |
| Yellow Card | A completed Yellow card can be submitted to CARM via email, fax or mail (address is on the card). |
| CARMReport@health.govt.nz |
Medsafe cannot give advice about an individual's medical condition. If you
have any concerns about a medicine you are taking Medsafe encourages you
to talk to your healthcare professional.





