Published: 17 June 2015
Revised: 29 August 2023
Safety Information
Trans-Tasman Early Warning System - Alert Communication
Ibuprofen and Cardiovascular Safety
Information for consumers and caregivers
Information for healthcare professionals
Review Summary
What actions are Medsafe taking?
How to report adverse events
Further information
Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) used for the relief of acute and/or chronic pain and inflammation.
Medsafe and the Medicines Adverse Reactions Committee (MARC) have concluded that there is a small increased risk of cardiovascular thrombotic events with ibuprofen, when used at high doses (2400 mg per day) and in long-term treatment. Overall, the studies suggest that lower doses of ibuprofen (1200 mg per day or less), the dose generally used for over-the-counter (OTC) preparations, are not associated with this increased cardiovascular risk.
Information for consumers and caregivers
- Non-steroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, are effective at relieving pain and inflammation (swelling).
- For most people, taking ibuprofen is safe. However, you
should check with your healthcare professional that this medicine
is appropriate if you:
- have high blood pressure
- have high cholesterol
- have diabetes
- are a smoker or
- have current or a history of heart failure or heart disease.
- Use the lowest dose that works for you and stop as soon as you can.
- Medsafe cannot give advice about an individual's medical condition. If you have any concerns about a medicine you are taking Medsafe encourage you to talk to your healthcare professional.
Information for healthcare professionals
- The overall benefit to risk of harm balance of ibuprofen remains positive.
- There is a small increased risk of cardiovascular events with ibuprofen, when used at high doses (2400 mg per day) and for long-term therapy.
- Prescribe the lowest effective dose for the shortest possible duration.
- All non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with an increase in cardiovascular risk. It is not possible to clearly differentiate cardiovascular risk between different NSAIDs.
- Patients receiving long-term ibuprofen should be periodically reviewed for effectiveness, adverse effects and development of cardiovascular risk factors.
- Relevant risk factors for cardiovascular events associated with high-dose ibuprofen (and other NSAIDs) include hypertension, hyperlipidaemia, diabetes and smoking.
- Prescribe with caution in patients with current or a history of heart failure, heart disease or circulatory problems such as stroke.
- Discuss the risks of harm and benefits of NSAID treatment with patients before commencing therapy.
- Report any adverse reactions to the Centre for Adverse Reactions Monitoring (CARM).
Review Summary
The Medicines Adverse Reactions Committee (MARC) reviewed the latest data on the association of adverse cardiovascular events in patients taking ibuprofen in March 2015. The MARC concluded that there was a small increase in the risk of cardiovascular events with the use of high dose ibuprofen (2400 mg per day).
The MARC noted that the published studies were observational, underpowered and confounded. Therefore, the conclusions that could be reached were limited.
There was a hint of a dose-response effect suggesting that there was no increased risk of myocardial infarction in doses less than or equal to 1200 mg daily. Available data on this risk with doses between 1200 mg and 2400 mg daily was insufficient to draw firm conclusions. There was evidence that there was an increased risk at a dose of 2400 mg daily.
Examples of data reviewed are shown below:
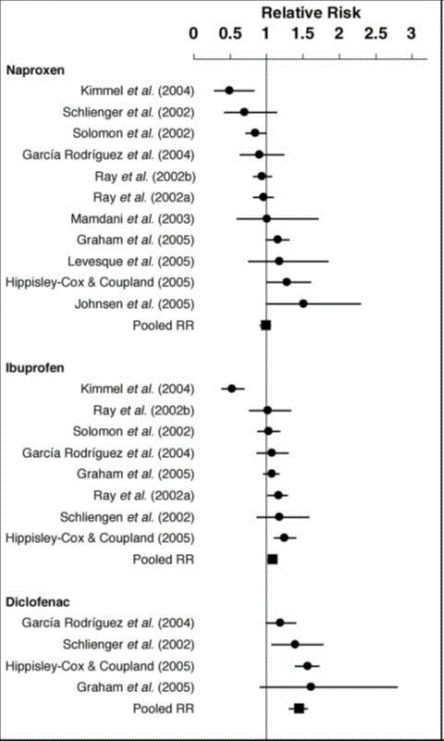
Figure 1: Pooled and individual relative risks and 95% confidence intervals for the risk of myocardial infarction associated with the use of naproxen, ibuprofen and diclofenac compared with no NSAID use1
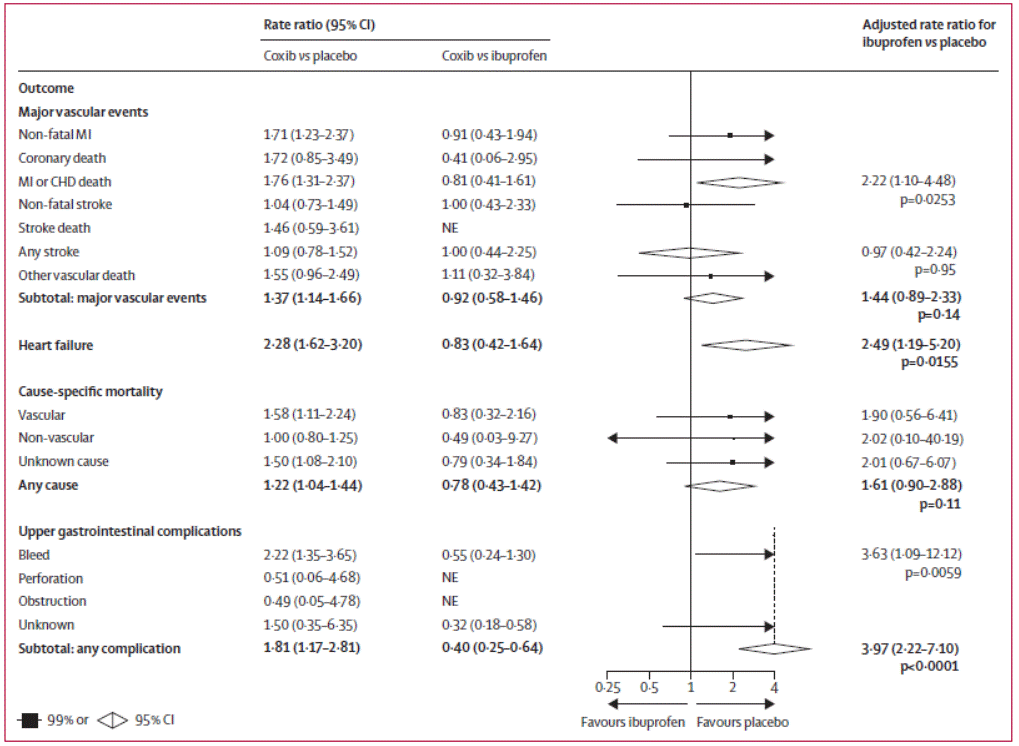
Figure 2: Effects of ibuprofen (2400 mg daily) on major vascular
events, heart failure, cause-specific mortality and upper gastrointestinal
complications2
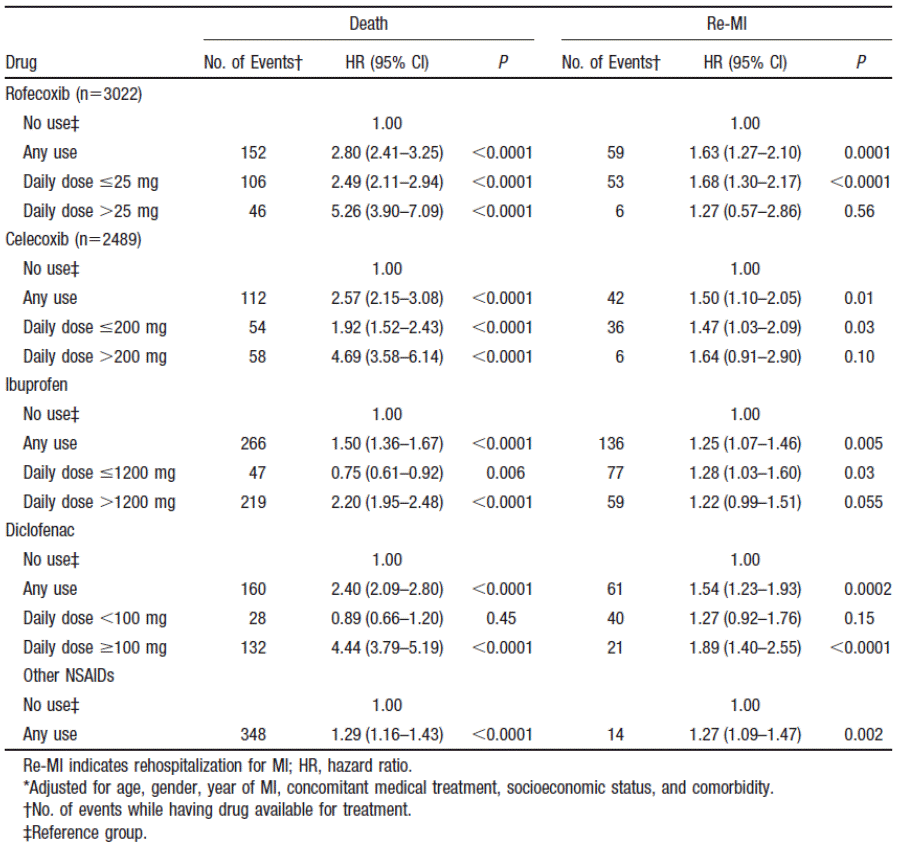
Table 1: Hazard ratios for death and rehospitalisation for myocardial infarction (Cox proportional hazards analysis)3
References
- Hernandez-Diaz S, Varas-Lorenzo C and Garcia Rodriguez LA. 2006. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol 98 (3): 266-274.
- Coxib and traditional NSAID Trialist’s (CNT) Collaboration. 2013. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382(9894): 769-779.
- Gislason GH, Jacobsen S, Rasmussen JN et al. 2006. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation 113(25): 2906-2913.
What actions are Medsafe taking?
Medsafe is working with the sponsors of these products to ensure data sheets and Consumer Medicine Information appropriately outline the safety of ibuprofen. No changes are being requested to over-the-counter (OTC) preparations.
How to report adverse events
| Online |
Submit a CARM report Prescribers can also submit a report using the online reporting tool available in patient management software. |
| Paper |
Download a consumer reporting form (Word Document, 61KB,
1 page) Download a healthcare professional reporting form (PDF, 292 KB, 2 pages) Submit completed forms by emailing CARMreport@health.govt.nz or mail (Medsafe, Ministry of Health, 133 Molesworth Street, Thorndon, Wellington, 6011). |
| CARMreport@health.govt.nz |
Medsafe cannot give advice about an individual’s medical condition.
If you have any concerns about a medicine you are taking Medsafe encourages
you to talk to your healthcare professional.





