Published: 22 December 2014
Revised: 29 August 2023
Safety Information
Trans-Tasman Early Warning System - Alert Communication
Domperidone (Motilium, Prokinex) – conclusion of review of benefits and risks of harm
Products affected
Information for consumers and caregivers
Information for healthcare professionals
Review Summary
What actions are Medsafe taking?
How to report adverse events
Further information
Domperidone is an anti-nausea medicine that has gastroprokinetic properties. Domperidone is used to treat:
- dyspeptic symptoms that may be associated with delayed gastric emptying (eg, epigastric sense of fullness, abdominal distention or swelling, or epigastric pain or discomfort), and
- acute symptoms of nausea and vomiting.
Recent research has suggested an increased risk of QT prolongation (alteration of the electrical activity of the heart), arrhythmias (unstable heartbeats) and sudden cardiac death with the use of oral domperidone.
Medsafe outlined these concerns in a monitoring communication published during March 2014. Due to these concerns, Medsafe and the Medicines Adverse Reactions Committee (MARC) reviewed the benefits and risks of harms of domperidone-containing medicines.
Monitoring communication: Domperidone (Motilium, Prokinex) and effects on the heart
Medicines Adverse Reactions Committee minutes of the 158th meeting
Medsafe and the MARC have concluded there is a small increased risk of adverse heart effects. However, the balance of benefits and potential harms for domperidone remains favourable. The available data suggests that this small increased risk may be higher in patients older than 60 years or at total daily doses of more than 30 mg.
Despite support from the MARC to maintain a maximum recommended daily dose of 80 mg, Janssen (manufacturer of the Motilium brand of domperidone) has concluded that the maximum recommended dose should be reduced from 80 mg to 40 mg daily. This dose change and other changes and additions to domperidone data sheets have been recommended to manage the potential risks to the heart associated with use of domperidone.
Products affected
| Product name | Sponsor |
|---|---|
| Motilium | Janssen-Cilag (New Zealand) Ltd |
| Prokinex | Air Flow Products Limited |
Information for consumers and caregivers
- For most people, taking domperidone is safe. However, if you have heart problems or are taking other medicines, you should first check with your healthcare professional that this medicine is appropriate.
- Use the lowest dose that works for you and stop as soon as you can.
- If you experience any symptoms such as dizziness, fainting or heart palpitations while you are taking domperidone, you should stop taking this medicine and seek medical attention. These symptoms may be due to an abnormal heart rhythm caused by domperidone.
- Medsafe cannot give advice about an individual’s medical condition. If you have any concerns about a medicine you are taking Medsafe encourages you to talk to your healthcare professional.
Information for healthcare professionals
- The overall benefit-risk balance of domperidone remains positive.
- The maximum recommended dose of domperidone has been reduced from 80 mg per day to 40 mg per day. Use the lowest effective dose for the shortest possible duration.
- The maximum recommended dose in children weighing ≤ 35 kg is 0.25 mg/kg three to four times daily up to 1.0 mg/kg/day. Domperidone is contraindicated in children younger than 2 years of age.
- Domperidone has been linked to QT interval prolongation and therefore is contraindicated with potent CYP3A4 inhibitors and with other medicines that prolong the QTc interval.
- Domperidone should be used with caution in older patients or those with current or history of cardiac disease.
- Patients receiving long-term therapy with domperidone should be regularly reviewed for effectiveness, adverse effects and development of cardiovascular risk factors.
- Relevant risk factors for cardiovascular events associated with domperidone include hypertension, hyperlipidaemia, obesity, diabetes, smoking and excessive alcohol consumption.
- Domperidone is indicated for dyspeptic symptoms associated with delayed gastric emptying and acute symptoms of nausea and vomiting.
- There is insufficient evidence to support the use of domperidone in childhood gastro-oesophageal reflux disease and it may not be suitable for chemotherapy- or radiotherapy-induced nausea and vomiting or post-operative nausea and vomiting.
- Other medicines used to treat digestive disorders or nausea and vomiting have also been found to cause QTc interval prolongation.
- Discuss the benefits and risk of harms of domperidone treatment with patients before commencing therapy. Report any adverse reactions to the Centre of Adverse Reactions Monitoring (CARM).
Review Summary
The MARC reviewed the latest data in June 2014. The MARC concluded that while the balance of benefits and risk of harms for domperidone remains favourable there is a small increased risk of adverse heart effects.
Many studies had a low number of overall events and the risk values varied between the different studies. Therefore, a true estimate of the risk could not be measured. A summary of the data reviewed is shown below:
Table 1: Risk of sudden cardiac death: population-based case-control study1.
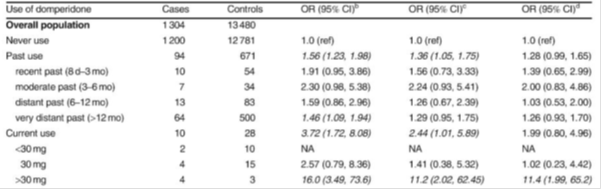
Table 2: Risk of serious ventricular arrhythmia and sudden cardiac death with domperidone relative to no exposure to study medicines or proton pump inhibitor exposure: population-based nested case-control study2.
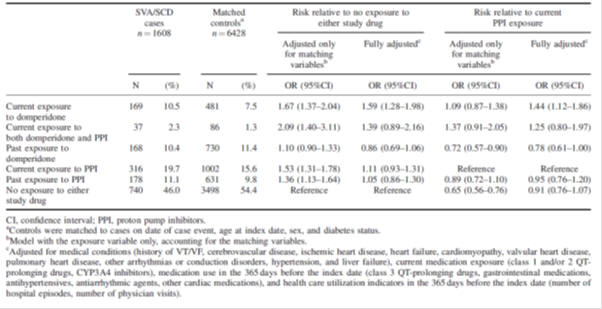
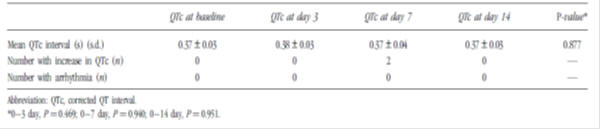
Table 3: Evaluation of QTc interval before and during domperidone administration in premature infants (n=40)3
There were limitations to the studies included in the review, such as the observational nature of most studies, a lack of information about indication for use, disease severity, dosage and regimen, and other risk factors such as smoking status, concomitant medicines and co-morbidities. Protopathic bias is when a treatment for the first symptoms of a disease or other outcome appear to cause the outcome. In this situation, patients may have been prescribed domperidone for non-specific gastrointestinal symptoms that may in fact be the prodromal symptoms of coronary heart disease. Therefore, protopathic bias and confounding by indication cannot be excluded in many of the observational studies.
Additional studies reviewed included a post-authorisation safety study as well as studies investigating the effects of domperidone use on the QTc interval in adults. A thorough QT study showed no clinically relevant dose-QTc response or exposure-QTc response with domperidone; however case reports of QTc prolongation, arrhythmia and sudden cardiac death with domperidone mean this risk cannot be completely excluded.
The MARC noted that other types of medicines used to treat digestive disorders or nausea and vomiting have also been associated with QT interval prolongation. These factors need to be taken into account when choosing the best medicine for an individual patient.
References
- van Noord C, Dieleman JP, van Herpen G, et al. (2010). Domperidone and Ventricular Arrhythmia or Sudden Cardiac Death: a population-based case-control study in the Netherlands. Drug Safety, 33(11): 1003-14
- Johannes CB, Varas-Lorenzo C, McQuay LJ, et al. (2010). Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: a nested case-control study. Pharmacoepidemiology and Drug Safety, 19: 881-88
- Gunlemez A, Babaoglu A, Arisoy AE, et al. (2010). Effect of domperidone on the QTc interval in premature infants. Journal of Perinatology, 30: 50-3
What actions are Medsafe taking?
Medsafe is working with the sponsors of these products to ensure data sheets and Consumer Medicine Information appropriately outline the safety of domperidone.
How to report adverse events
| Online |
Submit a CARM report Prescribers can also submit a report using the online reporting tool available in patient management software. |
| Paper |
Download a consumer reporting form (Word Document, 61KB,
1 page) Download a healthcare professional reporting form (PDF, 292 KB, 2 pages) Submit completed forms by emailing CARMreport@health.govt.nz or mail (Medsafe, Ministry of Health, 133 Molesworth Street, Thorndon, Wellington, 6011). |
| CARMreport@health.govt.nz |
Medsafe cannot give advice about an individual’s medical condition.
If you have any concerns about a medicine you are taking Medsafe encourages
you to talk to your healthcare professional.






