Published: 5 March 2020
Revised: 29 August 2023
Publications
Adverse reaction reporting in New Zealand – 2019
Prescriber Update 41(1): 8-9
March 2020
Thank you to everyone who submitted reports of suspected adverse reactions during 2019. You are making an important contribution to the safety monitoring of medicines in New Zealand. Reporting suspected adverse drug reactions enables Medsafe to quickly identify and respond to emerging medicine safety issues.
What is being reported?
In 2019, the Centre for Adverse Reactions Monitoring (CARM) received a total of 4,247 reports of suspected adverse reactions. These included 2,688 reports associated with medicines, 1,531 reports associated with vaccines and 28 reports associated with complementary or alternative medicines (CAMs). The reporting pattern is similar to previous years (Figure 1).
Figure 1: Adverse reactions to medicines, vaccines and CAMs, reported to the Centre for Adverse Reactions Monitoring, 2015–2019
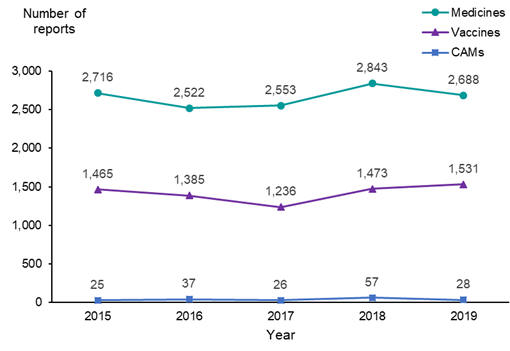
Of all reports received in 2019, 18.7 percent were considered serious. Serious reports accounted for 27.7 percent of medicine reports, 2.8 percent of vaccine reports and 28.6 percent of CAM reports. A serious adverse reaction is defined as any reaction that results in death or is life-threatening, causes or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital abnormality or is a medically important event.
You can find more information about suspected adverse reactions reported in New Zealand on the Medsafe website, using the Suspected Medicines Adverse Reaction Search (SMARS).
Who is reporting?
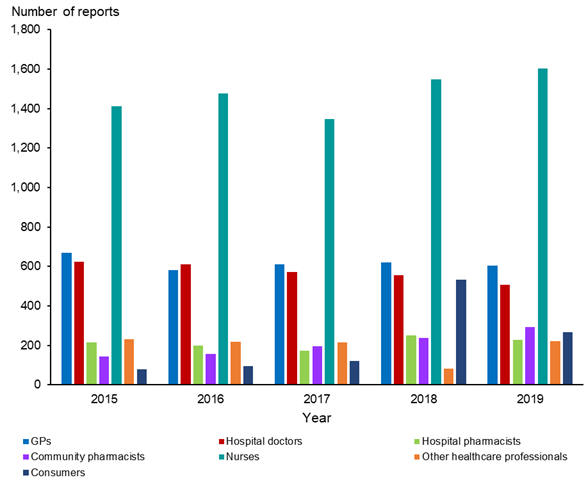
Anyone can submit a report. Figure 2 shows the number of reports received from health care professionals and consumers during the last five years. Nurses continue to submit the most reports.
Figure 2: Number of reports received from health care professionals and consumers, 2015–2019
Reporting is easiest online
Please continue to report any suspected adverse reactions to medicines, vaccines or CAMs to CARM. Online reporting is easiest.
Other ways of reporting include:
| Online |
Submit a CARM report Prescribers can also submit a report using the online reporting tool available in patient management software. |
| Paper |
Download a consumer reporting form (Word Document, 61KB,
1 page) Download a healthcare professional reporting form (PDF, 292 KB, 2 pages) Submit completed forms by emailing CARMreport@health.govt.nz or mail (Medsafe, Ministry of Health, 133 Molesworth Street, Thorndon, Wellington, 6011). |
| CARMreport@health.govt.nz |





