Published: 2 March 2017
Revised: 29 August 2023
Publications
Adverse Reaction Reporting in New Zealand – 2016
Prescriber Update 38(1): 8
March 2017
Medsafe and the Centre for Adverse Reactions Monitoring (CARM) would like to thank all those who have submitted reports of suspected adverse reactions in 2016. These reports make an important contribution to the post-market monitoring of medicines in New Zealand.
Medsafe uses this information to identify possible safety issues and take appropriate actions, such as:
- taking the issue to the Medicines Adverse Reaction Committee (MARC) for advice
- requesting updates to data sheets
- providing information through Prescriber Update
- further investigating the issue through the M² scheme
In 2016, CARM received a total of 3,944 reports of suspected adverse reactions. These reports included 2,522 reports associated with medicines, 1,385 reports associated with vaccines and 37 reports associated with a complementary or alternative medicine (CAM).
Of the reports received, 41% of medicine reports, 32.4% of CAM reports and only 3.6% of vaccine reports were considered serious. A serious adverse reaction is defined, according to internationally agreed criteria, as any reactions that result in death or is life-threatening, causes or prolongs hospitalisation, results in persistent or significant disability/incapacity or is a congenital abnormality.
Additional information about suspected adverse reactions reported in New Zealand can be found on the Medsafe website using the Suspected Medicines Adverse Reaction Search (SMARS) (www.medsafe.govt.nz/SMARS/).
Nurses continue to be the most frequent reporter in 2016, followed by hospital doctors and general practitioners (GPs) (Figure 1).
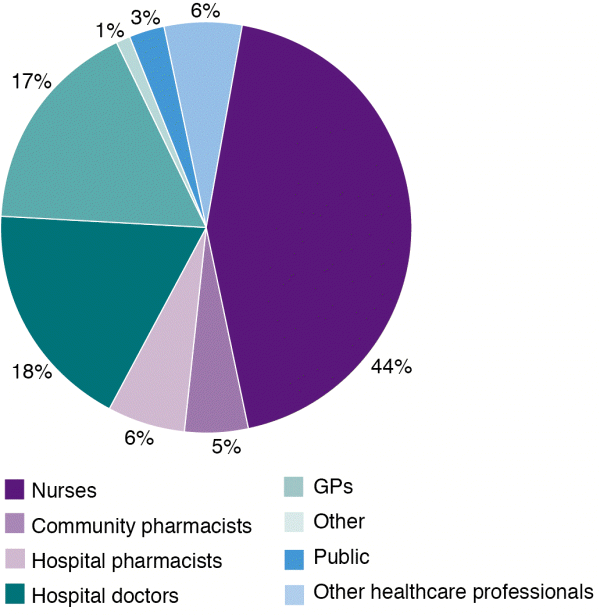
Figure 1: Source of adverse reaction reports in New Zealand in 2016
Healthcare professionals and consumers are encouraged to report any suspected adverse reactions to medicines, vaccines or CAMs to CARM.
Suspected adverse reactions can be reported using one of the following options.
| Online |
Submit a CARM report Prescribers can also submit a report using the online reporting tool available in patient management software. |
| Paper |
Download a consumer reporting form (Word Document, 61KB,
1 page) Download a healthcare professional reporting form (PDF, 292 KB, 2 pages) Submit completed forms by emailing CARMreport@health.govt.nz or mail (Medsafe, Ministry of Health, 133 Molesworth Street, Thorndon, Wellington, 6011). |
| CARMreport@health.govt.nz |





