Published: 1 June 2023
Publications
Autoimmune complications of immunotherapy
Published: 1 June 2023
Prescriber Update 44(2): 38–40
June 2023
The Centre for Adverse Reactions Monitoring (CARM) received a case report
(CARM ID 143560) of a patient diagnosed with type 1 diabetes mellitus
following treatment with pembrolizumab. This article is a reminder of
immune-related adverse events associated with immune checkpoint inhibitors.
Immune checkpoint inhibitors
Normally, the immune system uses a combination of co-inhibitory and co-stimulatory receptors and their ligands, known as immune checkpoints, to protect the body from infection, autoimmune disorders and allergies. However, by developing mechanisms to avoid them, cancer cells can evade these immune checkpoints and grow uncontrollably.1
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target and inhibit immune checkpoints. This inhibition stimulates the host's immune system to recognise and attack cancer cells.1 ICIs are indicated for the treatment of several advanced or metastatic cancers, and include pembrolizumab (Keytruda), durvalumab (Imfinzi), atezolizumab (Tecentriq), nivolumab (Opdivo) and ipilimumab (Yervoy).
Two key immune checkpoints targeted by immune checkpoint inhibitors are the cytotoxic T-lymphocyte antigen-4 (CTLA-4) receptor and the programmed death-1 (PD-1) receptor plus its ligand PD-L1.2 These immune checkpoints are self-recognition signal receptor systems that allow the body’s immune system to distinguish between its own cells and foreign pathogens. Cancer cells can exploit these immune checkpoints to reduce T-cell activity and thus weaken the immune system's response to the cancer.
Immune-related adverse events (irAEs)
By targeting the self-recognition signal receptor systems, immune checkpoint inhibitors remove one of the immune system’s safeguards. They increase T-cell activity against cancer cells, but ICIs may also increase the risk of T-cell attack on healthy cells, leading to immune-related adverse effects irAEs.2
Immune-related adverse effects associated with ICIs vary in severity and may affect different organ systems (Figure 1).
- Common irAEs include fatigue, rash, itching, diarrhoea and nausea.2
- Less common but potentially more serious irAEs can affect the lungs, liver, endocrine system (such as the thyroid and adrenal glands) and gastrointestinal system. These irAEs may include respiratory disorders, hepatitis, thyroiditis, colitis and pancreatitis.2
- Rare irAEs include neurological and blood disorders, and autoimmune conditions such as rheumatoid arthritis, type 1 diabetes and adrenal insufficiency.2
Figure 1: The spectrum of immune-related adverse events* by affected body system
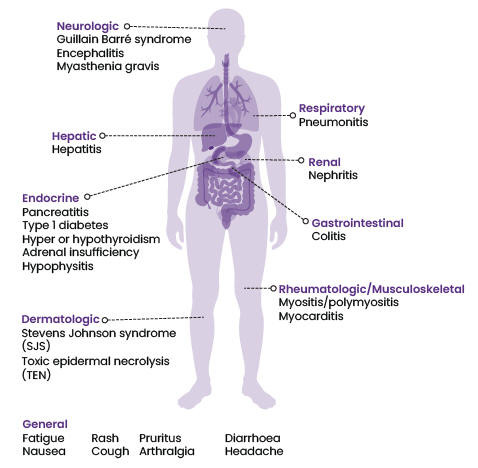
* Not a complete list. Refer to the data sheets for more information.
Sources: Keytruda, Imfinzi, Tecentriq, Opdivo and Yervoy data sheets, available at: medsafe.govt.nz/Medicines/infoSearch.asp (accessed 4 May 2023).
Monitoring for irAEs
Immune-related adverse events can occur during treatment with ICIs but may also occur after treatment stops. Clinicians should consider the possibility of irAEs in ICI-treated patients with any unexplained illness.1 The specific symptoms and signs of irAEs may indicate which organ system is affected, such as shortness of breath (respiratory), low libido (endocrine) or joint pain (musculoskeletal).2 Patients may also have abnormal blood test results, such as abnormal liver function tests, low cortisol levels or abnormal thyroid function tests.2
Rapid identification of irAEs can improve patient outcomes.2 Based on the severity of the irAE, withhold immune checkpoint inhibitor therapy and consider administering corticosteroids.3–7
Refer to the data sheets for further guidance.
References
- bpacNZ. 2018. Immune checkpoint inhibitors: a new cancer treatment. URL: bpac.org.nz/2018/checkpoint.aspx (accessed 16 October 2022).
- Pastow M and Johnson DB. 2023. Toxicities associated with checkpoint inhibitor immunotherapy. In: UpToDate 28 March 2023. URL: uptodate.com/contents/toxicities-associated-with-immune-checkpoint-inhibitors (accessed 19 April 2023).
- Merck Sharp & Dohme (New Zealand) Limited. 2022. Keytruda New Zealand Data Sheet 1 February 2023. URL: medsafe.govt.nz/profs/datasheet/k/Keytruda.pdf (accessed 19 April 2023).
- AstraZeneca Limited. 2023. Imfinzi New Zealand Data Sheet 21 March 2023. URL: medsafe.govt.nz/profs/datasheet/i/imfinziinf.pdf (accessed 19 April 2023).
- Roche Products (New Zealand) Limited. 2023. Tecentriq New Zealand Data Sheet 2 March 2023. URL: medsafe.govt.nz/profs/Datasheet/t/Tecentriqinf.pdf (accessed 4 May 2023).
- Bristol-Myers Squibb (NZ) Limited. 2022. Opdivo New Zealand Data Sheet 2 September 2022. URL: medsafe.govt.nz/profs/Datasheet/o/opdivoinf.pdf (accessed 4 May 2023).
- Bristol-Myers Squibb (NZ) Limited. 2022. Yervoy New Zealand Data Sheet 3 August 2022. URL: medsafe.govt.nz/profs/datasheet/y/yervoyinj.pdf (accessed 19 April 2023).





