Published: 6 December 2019
Publications
Photosensitivity reactions – The other side of summer
Prescriber Update 40(4): 73–74December 2019
As the weather gets warmer, consider the risk of photosensitivity reactions.
Photosensitivity reactions occur with a number of medicines and result
in sunburn or dermatitis on sun-exposed skin1,2.
Drug-induced photosensitivity reactions occur after exposure to a topical or systemic photosensitising medicine and exposure to ultraviolet (UV) or visible radiation1. The reactions can be classed as either phototoxic or photoallergic3.
Phototoxic reactions
Phototoxic reactions are more frequent than photoallergic reactions. They occur within minutes to hours after exposure to light and result from cellular damage1–3. Signs include an exaggerated sunburn reaction, vesicles, blisters and bullae2. The reaction is restricted to sun-exposed skin2.
Photoallergic reactions
Photoallergic reactions are immune-mediated and take a longer time (24–72 hours) to appear after exposure to light1,2. Photoallergic reactions are eczematous and itchy and may spread to non-exposed areas of skin2.
Treatment
The best treatment option is to avoid the photosensitising medicine2. If it is not possible to discontinue the medicine, the following sun protection strategies are recommended.
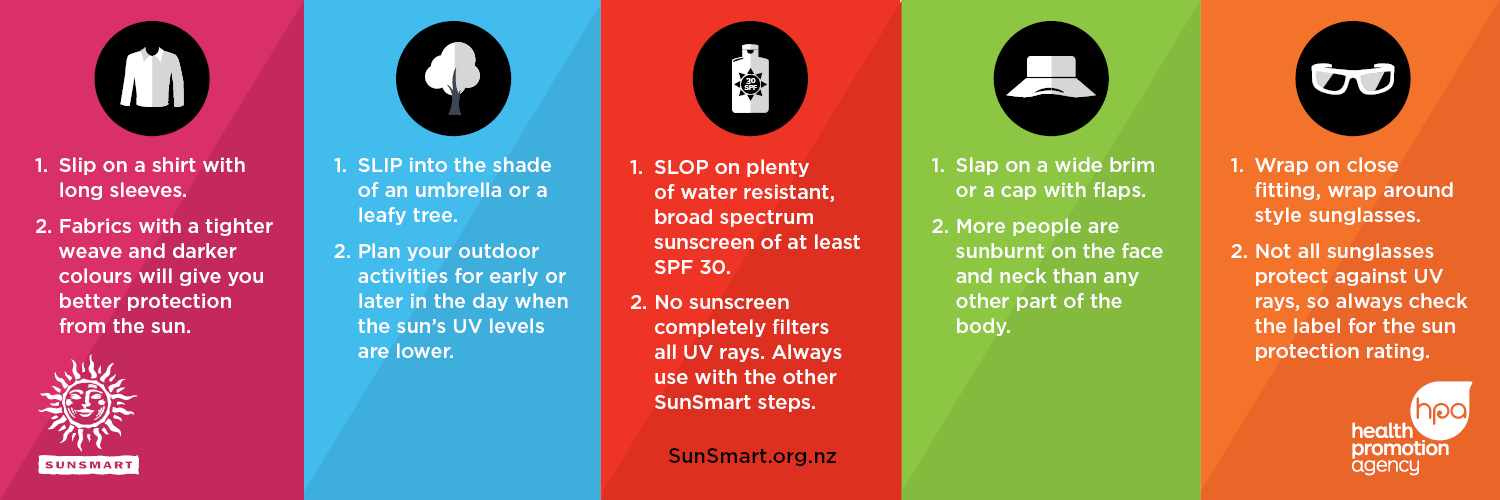
How to be SunSmart4
- Slip on a shirt/top with long sleeves and a collar.
- Slip into the shade.
- Slop on sunscreen that is at least SPF 30, broad-spectrum and water resistant. Apply 20 minutes before going outside and reapply every 2 hours.
- Slap on a broad-brimmed hat that shades the face, head, neck and ears.
- Wrap on close fitting sunglasses.
- Don’t use sunbeds.
Visit the SunSmart NZ website to learn more about staying safe in the sun.
What medicines cause photosensitivity?
A variety of medicines have been associated with photosensitivity reactions including antibiotics, cancer medicines, cardiovascular medicines, some decongestants and antihistamines, medicines to treat diabetes, diuretics, hormones, nonsteroidal anti-inflammatory drugs (NSAIDs), psychiatric medicines and retinoids2,5. See the DermNet NZ website for a list of photosensitising medicines.
The arrival of summer is a good opportunity to review any new medicines started by your patients for photosensitivity. For example, vemurafenib (brand name Zelboraf) is a relatively new medicine that has been associated with mild to severe photosensitivity6. Patients should avoid sun exposure when taking Zelboraf6.
Refer to the medicine data sheet for further information on photosensitivity reactions for each specific medicine.
References
- Blakely KM, Drucker AM and Rosen CF. 2019. Drug-Induced Photosensitivity-An Update: Culprit Drugs, Prevention and Management. Drug Saf 42(7): 827-47. DOI: 10.1007/s40264-019-00806-5 (accessed 15 October 2019).
- DermNet NZ. 2006. Drug-induced photosensitivity. URL: www.dermnetnz.org/topics/drug-induced-photosensitivity/ (accessed 15 October 2019).
- Khandpur S, Porter RM, Boulton SJ, et al. 2017. Drug-induced photosensitivity: new insights into pathomechanisms and clinical variation through basic and applied science. Br J Dermatol 176(4): 902-09. DOI: 10.1111/bjd.14935 (accessed 15 October 2019).
- Health Promotion Agency and Melanoma Network of New Zealand (MelNet). 2019. New Zealand Skin Cancer Primary Prevention and Early Detection Strategy 2017–2022. URL: www.sunsmart.org.nz/sites/default/files/documents/1.0%20SS115%20Skin%20Cancer%20Strategy_Feb%202019_Online.pdf (accessed 20 November 2019).
- Harvard Women's Health Watch. 2017. 10 types of medications that should keep you in the shade this summer July 2017. URL: www.health.harvard.edu/skin-and-hair/10-types-of-medications (accessed 17 October 2019).
- Roche Products (New Zealand) Limited. 2017. Zelboraf New Zealand Data Sheet 5 September 2017. URL: www.medsafe.govt.nz/profs/Datasheet/z/zelboraftab.pdf (accessed 17 October 2019).





