Published: 3 December 2020
Publications
Acute generalised exanthematous pustulosis (AGEP)
Published: 3 December 2020
Prescriber Update 41(4): 81
December 2020
What is AGEP?
Acute generalised exanthematous pustulosis (AGEP) is a rare type of severe cutaneous adverse reaction (SCAR).1,2 Like other SCARs, such as Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS), AGEP is a type IV (T-cell mediated) hypersensitivity reaction.1–3 The T-cell mediated response in AGEP results in neutrophilic inflammation,1,2 but there can be some overlap between the different types of SCAR and patients may have clinical features of SJS/TEN or DRESS with AGEP.2–4
How does AGEP present?
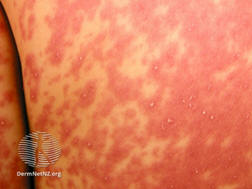
AGEP is characterised by the rapid appearance of sterile, non-follicular, pin-head sized pustules on an erythematous base (Figure 1), typically starting on the face and skin flexures before becoming more widespread.4,5 Over 90 percent of cases of AGEP are provoked by medicines.4 The rash usually develops rapidly, within two days of exposure to the offending medicine,2,4 but onset may occur up to two weeks after initial exposure to some medicines.2,3
In the acute phase, patients may have a fever above 38°C and an elevated neutrophil count,2,5,6 but internal organ involvement is uncommon.2,4
Most patients recover from AGEP within 10–14 days of withdrawal of the causative medicine.1,4–7 The pustular eruptions desquamate during the recovery phase.2,6 The mortality rate from AGEP is approximately 2–4 percent.1,3
Figure 1: Patient with acute generalised exanthematous pustulosis (AGEP), showing pinpoint pustules on an erythematous base
Source:
DermNet NZ. URL:
dermnetnz.org/imagedetail/3457?copyright=&label= (accessed 21 October
2020). No changes were made to this watermarked image.
Creative Commons Attribution-NonCommercial-NoDerivs 3.0 (New Zealand).
URL:
creativecommons.org/licenses/by-nc-nd/3.0/nz/legalcode.
How is AGEP managed?
Treatment primarily involves withdrawal of the causative medicine. Supportive care in hospital (including fluid and electrolyte replacement) is often required during the acute illness.2,4 Moisturisers, topical corticosteroids, oral antihistamines and analgesics may be required for symptomatic relief.2,4 Systemic corticosteroids may be used to control inflammation in the acute phase.2
Patients with AGEP should avoid re-exposure to the causative medicine.2
Which medicines are associated with AGEP?
Antibiotics are among the most commonly implicated medicines. Other medicines associated with AGEP include oral antifungals (eg, terbinafine), calcium channel blockers (eg, diltiazem), hydroxychloroquine, carbamazepine and paracetamol.4 Ibuprofen has recently been highlighted as a causative agent for AGEP.8
As at 1 October 2020, the Centre for Adverse Reactions Monitoring (CARM) has received 25 reports of AGEP, of which five reports included more than one suspect medicine. The reported medicines are shown in Table 1.
Table 1: Medicines reported in association with acute generalised exanthematous pustulosis (AGEP), December 2000 to October 2020
| Suspect medicine | No. of reports |
|---|---|
| Antibiotics: | |
| Penicillins | 5 |
| Cephalosporins | 2 |
| Clindamycin | 2 |
| Tetracyclines | 2 |
| Co-trimoxazole | 2 |
| Trimethoprim | 1 |
| Ciprofloxacin | 1 |
| Terbinafine | 3 |
| Hydroxychloroquine | 3 |
| Diltiazem | 3 |
| Contrast media | 2 |
| Other: | |
| Allopurinol | 1 |
| Bendamustine | 1 |
| Ibuprofen | 1 |
| Ondansetron | 1 |
| Rituximab | 1 |
References
- Bellón T. 2019. Mechanisms of severe cutaneous adverse reactions: recent advances. Drug Safety 42(8): 973–92. DOI: 10.1007/s40264-019-00825-2 (accessed 29 June 2020).
- Hadavand MA, Kaffenberger B, Cartron AM, et al. 2020. Clinical presentation and management of atypical and recalcitrant acute generalized exanthematous pustulosis (AGEP). Journal of the American Academy of Dermatology [In press]. DOI: 10.1016/j.jaad.2020.09.024 (accessed 16 October 2020).
- Nguyen DV, Vidal C, Chu HC, et al. 2019. Human leukocyte antigen-associated severe cutaneous adverse drug reactions: from bedside to bench and beyond. Asia Pac Allergy 9(3): e20. DOI: doi.org/10.5415/apallergy.2019.9.e20 (accessed 14 July 2020).
- Purvis D and Oakley A. 2015. Acute generalised exanthematous pustulosis. In: DermNet NZ September 2015. URL: dermnetnz.org/topics/acute-generalised-exanthematous-pustulosis/ (accessed 15 October 2020).
- Sidoroff A, Halevy S, Bavinck JN, et al. 2001. Acute generalized exanthematous pustulosis (AGEP) – a clinical reaction pattern. Journal of Cutaneous Pathology 28(3): 113–19. DOI: doi.org/10.1034/j.1600-0560.2001.028003113.x (accessed 16 October 2020).
- Guvenir H, Arikoglu T, Vezir E, et al. 2019. Clinical phenotypes of severe cutaneous drug hypersensitivity reactions. Current Pharmaceutical Design 25(36): 3840–54. DOI: 10.2174/1381612825666191107162921 (accessed 16 October 2020).
- Mockenhaupt M. 2009. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 7(2): 142–62. DOI: doi.org/10.1111/j.1610-0387.2008.06878.x (accessed 16 October 2020).
- European Medicines Agency. 2019. Pharmacovigilance Risk Assessment Committee (PRAC) recommendations on signals – Adopted at the 2–5 September 2019 meeting. URL: ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-2-5-september-2019-prac-meeting_en.pdf (accessed 21 October 2020).





