Published: 27 November 2020
Revised: 6 June 2024
Archived: 21 August 2024
About Medsafe
COVID-19 Archive
Vaccine Safety Monitoring Process
- How is Medsafe monitoring COVID-19 vaccine safety?
- Identifying and investigating potential safety signals
- Reporting adverse events following immunisation
- More information
How is Medsafe monitoring COVID-19 vaccine safety?
Medsafe uses a variety of methods to collect information on the safety of vaccines after they have been approved for use. These methods, together with investigation of the information gathered and taking action when appropriate, are known as pharmacovigilance.
Along with our usual pharmacovigilance methods for monitoring vaccine safety, we are monitoring COVID-19 vaccine safety through:
- the spontaneous reporting system
- the Post Vaccine Symptom Check
- observed versus expected analysis
- rapid cycle analysis
- expert advice from the COVID-19 Vaccine Independent Safety Monitoring Board (CV-ISMB)
- information from other sources.
We use this information to identify and investigate potential safety signals for the COVID-19 vaccines. See below for an explanation of each of these methods.
Spontaneous reporting system
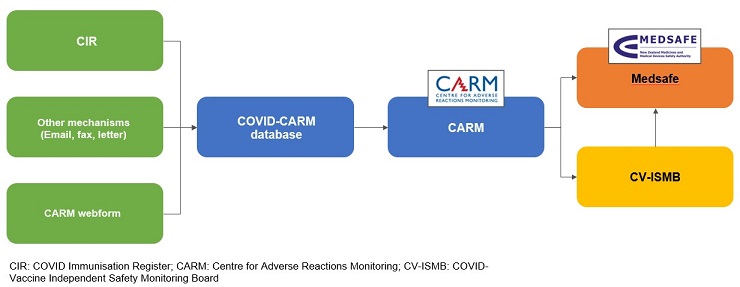
The spontaneous reporting system collects and monitors adverse events following immunisation (AEFIs) reported to the Centre for Adverse Reactions Monitoring (CARM) – see Figure 1.
An AEFI is an untoward medical event which follows immunisation and does not necessarily have a causal relationship with the administration of the vaccine. The adverse event may be an unfavourable or unintended sign, abnormal laboratory finding, symptom or disease.
Figure 1 below shows the COVID-19 vaccine spontaneous reporting system. AEFIs can be reported through the COVID Immunisation Register (CIR), by email, fax or letter or the online reporting form. Anyone can report an AEFI.
Reports are stored in the COVID-CARM database and then assessed by CARM, Medsafe and the CV-ISMB, as appropriate.
Figure 1 – COVID-19 vaccine spontaneous reporting system
Post Vaccine Symptom Check
Read about the Post Vaccine Symptom Check
Observed versus expected analyses
Observed versus expected analyses help us to determine if further action is necessary for a particular safety concern. We obtain the observed number of cases from AEFI reports and compare these with the expected number of cases, which is calculated based on background rates from independent sources, such as epidemiological studies or national statistics (eg, hospitalisation or mortality data from the Ministry of Health’s National Collections).
The comparison is done by dividing the observed rate by the expected rate to give the relative risk (RR).
Observed / Expected Rate = Relative Risk (RR)
The methods used to calculate the relative risk also provide a confidence interval (CI). The confidence interval is a range of values that we are fairly sure our true value lies in. We use a 95 percent confidence interval (95% CI), which is the range that will include the true value 95 percent of the time.
If both the relative risk AND the lower end of the confidence interval are greater than one (>1.0), this is statistically significant and could indicate an increased risk of that event in the vaccinated population.
Note that no conclusions should be made from observed-versus-expected analyses in isolation. Other investigations, such as looking at pre-existing risk factors, are always required.
Rapid cycle analysis
Rapid cycle analysis (RCA) is a type of observed versus expected analysis used as a signal detection tool. This analysis looks at baseline rates for Adverse Events of Special Interest (AESIs) (eg, death, myocarditis and thrombosis) and compares this to the rates of those AESIs within the vaccinated population. The data for this analysis is obtained from hospital discharge records from all over New Zealand.
RCA can provide an early warning signal when the vaccine is possibly linked with an AESI, so we can quickly investigate and take action as needed.
COVID-19 Vaccine Independent Safety Monitoring Board (CV-ISMB)
The CV-ISMB is an independent panel of experts in various fields of clinical medicine, biostatistics, microbiology and immunology. There is also a position for a lay member (non-healthcare professional) to represent the interests of the consumer.
The CV-ISMB provides advice on:
- interpretation of the information for reported AEFI
- significance of the information in relation to risk/benefit for signal review
- ensuring that equity remains a key consideration in the collection, monitoring and reporting of AEFI
- guidance and oversight of procedures for safety monitoring.
Information from other sources
We use information from many other sources to monitor COVID-19 vaccine safety, including:
- clinical and epidemiological studies
- case reports
- published medical literature
- pharmaceutical companies
- other regulatory authorities such as the Food and Drug Administration (USA), European Medicines Agency (Europe) and Therapeutic Goods Administration (Australia).
Identifying and investigating potential safety signals
A potential safety signal occurs when we receive information on a new or known side effect that may be caused by the vaccine and requires further investigation.
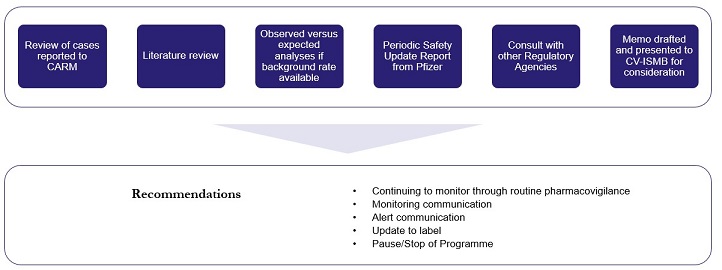
Safety signals can be detected from a wide range of sources such as CARM reports, clinical studies and the scientific literature – see Figure 2. Our process for investigating a potential COVID-19 vaccine safety signal is shown in Figure 3.
Figure 2: How Medsafe identifies potential safety signals
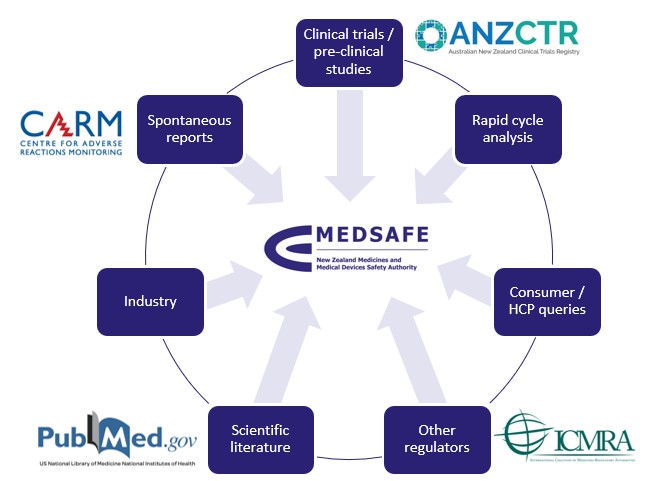
Figure 3: Medsafe’s process for investigating a potential COVID-19 vaccine safety signal
In our regular COVID-19 vaccine Safety Reports, we include summaries of the safety signals that we’ve investigated so far and the outcome.
Reporting adverse events following immunisation
Anyone living in New Zealand who thinks they may have experienced an AEFI can report it.
Reports may describe:
- real adverse drug reactions to the vaccine
- anxiety events or nervousness about needles or the process of vaccination
- coincidental events that would have occurred anyway.
More information
- COVID-19 vaccines (Ministry of Health)
- COVID-19 vaccine safety: questions and answers
- How Medsafe monitors vaccine safety
- How Medsafe monitors medicine safety
- Adverse reactions to medicines
- Reporting side effects of medicines (PDF, 237 KB, 1 page)
- COVID-19 vaccine evaluation and approval process
- ICMRA statement for healthcare professionals: How COVID-19 vaccines will be regulated for safety and effectiveness





