Published: 1 September 2016
Revised: 4 September 2025
Publications
About the Medication Error Reporting Programme (MERP)
Prescriber Update 37(3): 40
September 2016
Medication errors are unintentional errors in the use of a medicine. Errors
may arise at any stage of medicine use; prescribing, dispensing, administration
and monitoring. Errors include those of commission (where an error occurs
as a result of an action taken) and those of omission (where an error occurs
as a result of an action not taken). Medication errors occur across all
healthcare settings. Fortunately, most errors are detected and corrected
before reaching the patient. However, some errors are not and may result
in serious patient harm.
The Medication Error Reporting Programme (MERP) is an initiative of the New Zealand Pharmacovigilance Centre (NZPhvC) and operates alongside the Centre for Adverse Reactions Monitoring (CARM). The MERP aims to improve the safe use of medicines for New Zealanders.
The MERP is an online, national, voluntary, no-blame reporting system for primary care, where reports can be made anonymously if preferred. Voluntary reporting of actual and ‘near miss’ errors can help to identify hazards and risks. This information highlights vulnerabilities in systems that can be targeted for improvement to reduce the risk of harm to patients.
Importance of the MERP and synergy with CARM
Beyond local systems for managing medication errors, sharing events with the NZPhvC contributes to a national database of events which:
- complements the CARM programme, as harms from medicines may arise not only from adverse reactions but also errors in use
- helps identify rare events that may otherwise go unrecognised
- provides timely insights into New Zealand-specific error trends and patterns over time
- informs national medication safety initiatives.
How reporting to the MERP helps toward improving medication safety in New Zealand
The NZPhvC works with the Health Quality & Safety Commission (the Commission) to improve medication safety in New Zealand. Reports to the MERP are utilised to inform and drive national initiatives through a continual process involving reporting, analysis, solution development and systems change as shown in Figure 1.
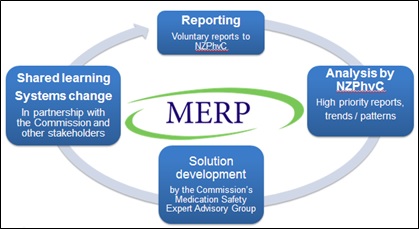
Figure 1: The MERP national reporting and learning system for primary care
Who can report to the MERP?
- Any healthcare professional can report a primary care medication error electronically.
- Organisations who collate data on medication errors can contribute to the MERP.
What to report to the MERP
- Actual and 'near miss' errors pertaining to prescribing, product labelling/packaging/names, compounding, dispensing, distribution, administration and monitoring. Reports of 'near misses' or unsafe situations that could lead to patient harm are encouraged as this allows a more proactive approach to prevention.
- Reporting of errors covers all types of medicines, vaccines, as well as complementary and herbal remedies, but excludes blood products.
Report a suspected medicine-related reaction, error or safety problem to the NZPhvC.
If unsure, please report to CARM or phone for advice.





