Published: 2 June 2016
Publications
Medicine-induced Lung Disease
Prescriber Update 37(2): 24-26
June 2016
Background
The most common form of drug-induced lung injury (DLI) is interstitial lung disease (also called interstitial pneumonia or interstitial pneumonitis)1. Interstitial lung disease (ILD) is an umbrella term for a large group of lung diseases that cause scarring of lung tissue through inflammation and fibrosis2. Medicines, herbal medicines, supplements and recreational drugs can all cause DLI3,4.
Incidence
The exact frequency of DLI is unknown, but it is probably underdiagnosed worldwide3. However, the incidence of lung adverse effects in patients taking amiodarone is around 5%5.
Diagnosis
Recognition of DLI is difficult as the symptoms and clinical, radiological and histological findings are often non-specific3. No clinical disease types are specific to DLI. A medicine may induce lung injuries characteristic of different clinical diseases in different patients3. In addition, the same clinical disease can be induced by more than one medicine2,6. To complicate matters further, medicines that can cause DLI are often used to treat conditions associated with lung disease1.
Patients typically present with dyspnoea, cough (often exacerbated by gastro-oesophageal reflux), general malaise and constitutional upset. The time course over which symptoms develop can sometimes help to differentiate the types of disease6.
Diagnosis of DLI is mainly one of exclusion. Differential diagnoses include chronic obstructive pulmonary disease, bronchitis, emphysema, asthma, infection, heart disease and idiopathic ILD1.
Diagnostic criteria for DLI include:1,3,4
- a history of ingestion of a medicine known to cause lung injury
- the clinical manifestations have been reported in association with the medicine
- other causes of lung disease have been ruled out (where possible)
- improvement following discontinuation of the suspected medicine (dependent on injury and medicine)
- exacerbation of clinical manifestations following re-exposure to the suspected medicine (not generally recommended).
Diagnosis may take over one year from the onset of breathing problems2.
Causal Medicines
Over 450 drugs have been implicated with ILD1,6. A list of these and the types of lung toxicity they are known to cause can be found at www.pneumotox.com
The more common medicines and lung injuries are outlined in Table 1.
Table 1: Examples of medicines and their pattern of induced lung injury1,4
| Pattern of Lung Injury | Medicines Commonly Implicated |
|---|---|
| Acute interstitial pneumonia/diffuse alveolar damage | Amiodarone, amphotericin B, azathioprine, bleomycin cetuximab, cyclophosphamide, erlotinib, etanercept, gefitinib, gold, infliximab, interferons, methadone, methotrexate, nitrofurantoin, panitumumab, phenytoin, rituximab, statins, sulfasalazine |
| Organising pneumonia/bronchiolitis obliterans with organising pneumonia | Amiodarone, bleomycin, cyclophosphamide, gold, methotrexate, penicillamine, phenytoin |
| Non-specific interstitial pneumonia | Amiodarone, gold, hydralazine, methotrexate |
| Hypersensitivity pneumonia/pneumonitis | Azathioprine, beta-blockers, fluoxetine, gefitinib, nitrofurantoin |
| Eosinophilic pneumonia | Amiodarone, aspirin, azathioprine, carbamazepine, clarithromycin, contrast media, diclofenac, G-CSF, gold, levofloxacin, methotrexate, minocycline, naproxen, paracetamol, penicillamine, penicillins, phenytoin, simvastatin |
Other non-medicine causes of DLI include talc and cocaine6.
Risk Factors
The likelihood of developing adverse pulmonary effects secondary to medicines remains largely unpredictable and idiosyncratic1. However, possible risk factors include:1
- smoking
- age (risk is increased in childhood and old age)
- ethnicity (higher rates are reported in Japan)
- dose (eg, amiodarone7 and bleomycin)
- pre-existing lung disease
- interactions (eg, the use of radiation therapy with bleomycin or contrast media with amiodarone)5.
Mechanisms
The mechanisms of DLI are unknown, but may include:
- a direct toxic effect due to high local concentrations of the medicine or the large surface area of the lungs1
- lung-specific metabolism of a medicine to a toxic metabolite1
- immune activation, if the medicine mimics an antigen or acts as a hapten1,4
- deposition of phospholipids within cells (eg, amiodarone causes phospholipidosis)1,4.
Management
The primary goal of treatment is to suppress the inflammatory response and prevent the deposition of fibrotic tissue. Failure to appreciate the relationship between the medicine and lung injury may lead to significant morbidity or death1.
Therefore, any medicine that is suspected of causing a DLI should be discontinued immediately, unless the benefits clearly outweigh the risks of DLI. Discontinuation in mild cases can be followed by spontaneous improvement and no further management is required1.
Patients with moderate to severe DLI should also receive steroids and supportive treatment4.
If the patient requires continued treatment, it is recommended to switch to a medicine less likely to cause lung injury, if possible4.
Prognosis
If DLI is diagnosed early, the patient may make a full recovery. Delayed diagnosis can lead to significant morbidity or death. This is related to the degree of fibrosis and comorbidity rather than severity of the initial clinical presentation. As an example, the overall mortality in patients with amiodarone DLI is less than 10%, but rises to 20% to 33% if the diagnosis is delayed1.
New Zealand Cases
The Centre for Adverse Reactions Monitoring (CARM) has received 296 reports of DLI. These reports involved 341 suspected medicines as more than one suspected medicine was described in some reports. The most frequently reported medicines are shown in Figure 1.
The average age of the patients experiencing a DLI was 67 years. The youngest patient was 16 years and the oldest was 97 years.
Time to onset was reported for 280 of the 341 suspected medicines. For eight of the suspected medicines, the onset was reported to have occurred within one day of treatment initiation. For 100 of the suspected medicines, the onset was longer than one year.
In 95 of the 296 cases, the patient was reported to have fully recovered, while 38 cases reported a fatal outcome.
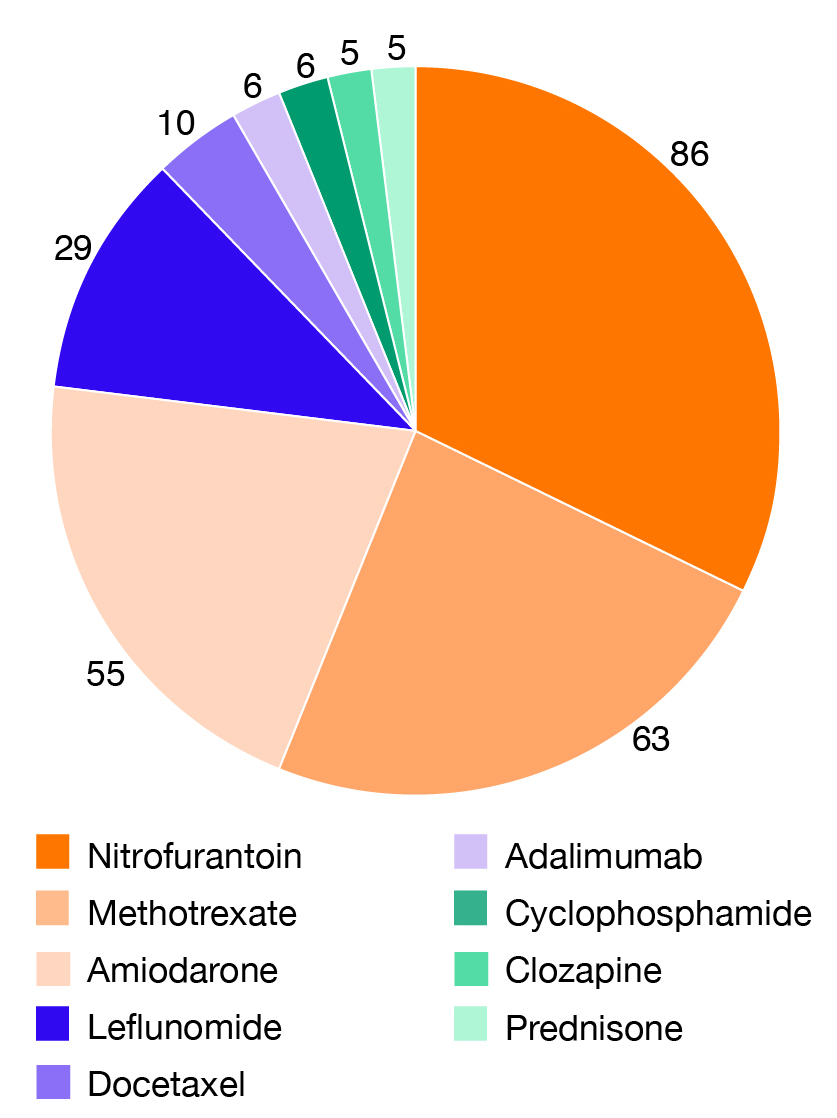
Figure 1: Medicines most frequently associated with DLI in the CARM database
References
- Schwaiblmair M, Behr W, Haeckel T, et al. 2012. Drug induced interstitial lung disease. The Open Respiratory Medicine Journal 6: 63-74.
- Zibrak JD, Price D. 2014. Interstitial lung disease: raising the index of suspicion in primary care. NPJ Primary Care Respiratory Medicine 24: 14054.
- Camus P. 2013. The Drug-Induced Respiratory Disease Website. Pneumotox Online v2.0. URL: www.pneumotox.com/pattern/index/I/ (accessed 18 April 2016).
- Kubo K, Azuma A, Kanazawa M, et al. 2013. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respiratory Investigation 51: 260-277.
- Papiris SA, Triantafillidou C, Kolilekas L, et al. 2010. Amiodarone: review of pulmonary effects and toxicity. Drug Safety 33: 539-558.
- Wallis A. 2015. The diagnosis and management of interstitial lung diseases. BMJ 350: h2072.
- Medsafe. 2013. Amiodarone Pulmonary Toxicity – early recognition is vital. Prescriber Update 34(4): 38-39. URL: www.medsafe.govt.nz/profs/PUArticles/December2013Amiodarone.htm (accessed 18 April 2016).





