Published: 13 December 2017
Publications
Update: Varenicline and Neuropsychiatric Adverse Reactions
Prescriber Update 38(4): 59-60
December 2017
Recently, the US Food and Drug Administration (FDA) announced they would
be removing the black box warning for serious neuropsychiatric adverse reactions
from the varenicline (Champix) data sheet1.
This decision is based on the results of a large clinical trial that evaluated
the neuropsychiatric safety of varenicline for smoking cessation in patients
with and without a history of psychiatric disorders2.
In the group of patients without a history of psychiatric disorder, 1.3% of the participants in the varenicline group reported moderate and severe neuropsychiatric adverse events, compared to 2.4% in the placebo group and 2.5% in the nicotine patch group2.
In the group of patients with a clinically stable psychiatric disorder, moderate and severe neuropsychiatric adverse events were reported in 6.5% of the varenicline group, compared with 4.9% in the placebo group and 5.2% in the nicotine patch group2.
Varenicline, bupropion, and nicotine replacement patches were all more effective for helping people quit smoking than placebo regardless of whether or not they had a history of mental illness2.
The data sheet for Champix notes that there have been reports of neuropsychiatric symptoms, as well as worsening of pre-existing psychiatric illness in patient’s attempting to quit smoking by taking Champix3.
Despite the recent study results, healthcare professionals are reminded to discuss with patients the benefits and risks of using varenicline as an aid to quit smoking. Patients and their families should also be advised to stop taking varenicline and contact their doctor or emergency services immediately if they notice changes in their mood, behaviour or thinking.
In the last five years (1 September 2012 to 31 August 2017), the Centre for Adverse Reactions Monitoring (CARM) has received 413 reports of suspected adverse reactions to varenicline, containing 762 reactions in total. The most frequently reported reaction was nausea (124). Of the 413 reports, 221 contained at least one neuropsychiatric reaction (361 neuropsychiatric reactions in total).
The most frequently reported neuropsychiatric reactions are shown in Table 1. There were 136 reports in females (61.5%) and 85 reports in males (38.5%). The majority of the reports were classified as not serious (90.5%). The most frequently reported age-group was 40–49 years (31.2%) (Figure 1).
Table 1: Most frequently reported suspected neuropsychiatric reactions during the period 1 September 2012 to 31 August 2017.
| Neuropsychiatric reaction | Frequency (Percentage of total reports) |
|---|---|
| Dreaming abnormal | 46 (6.04%) |
| Depression | 41 (5.38%) |
| Insomnia | 30 (3.94%) |
| Nightmares | 25 (3.28%) |
| Sleep disturbed | 17 (2.23%) |
| Anxiety | 16 (2.1%) |
| Suicidal ideation | 15 (1.97%) |
| Aggressive reaction | 14 (1.84%) |
| Anger | 12 (1.57%) |
| Emotional lability | 12 (1.57%) |
| Irritability | 12 (1.57%) |
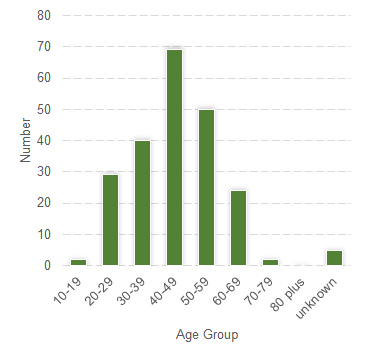
Figure 1: Suspected adverse reaction reports to varenicline containing neuropsychiatric reactions submitted to CARM during the period 1 September 2012 to 31 August 2017, by age group.
Information on how to report suspected adverse reactions to medicines can be found on the Medsafe website (www.medsafe.govt.nz/safety/report-a-problem.asp) or the CARM website (https://nzphvc.otago.ac.nz/reporting/).
References
- Food and Drug Administration. 2016. FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. Drug Safety Communications 16 December 2016.
- Anthenelli RM, Benowitz NL, West R, et al. 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 387: 2507–20.
- Pfizer New Zealand Limited. 2017. Champix Data Sheet 15 March 2017. URL: medsafe.govt.nz/profs/Datasheet/c/Champixtab.pdf (assessed 15 November 2017).





