Published: December 2011
Publications
Dabigatran etexilate (Pradaxa): Summary of reports to CARM
Prescriber Update 32(4): 29-30
December 2011
The funding of Pradaxa by Pharmac on 1 July 2011 has created a great deal of interest among healthcare professionals and the public. In turn this has stimulated the reporting of suspected adverse reactions associated with Pradaxa to the Centre for Adverse Reactions Monitoring (CARM).
Early data indicates that about 10,000 patients started dabigatran treatment up until the end of September 2011.
As of 7 November 2011 there were 295 reports in the CARM database detailing suspected adverse reactions to dabigatran. These reports were distributed across the different body systems as outlined in table 1. A suspected adverse reaction does not necessarily mean that the medicine caused the reaction, merely that the reporter suspected it may have.
Table 1: Distribution of reported suspected reactions to dabigatran
| Category# | Total number of reactions* |
|---|---|
| Alimentary | 256 |
| Application site | 1 |
| Cardiovascular | 41 |
| Endocrine/Metabolic | 8 |
| Haematological | 27 |
| Musculoskeletal | 14 |
| Nervous System | 37 |
| Other | 28 |
| Procedure Related | 20 |
| Product Related | 2 |
| Psychiatric Changes | 35 |
| Reproductive disorders | 1 |
| Resistance Mechanism Disorders | 2 |
| Respiratory | 31 |
| Skin & appendages | 28 |
| Special Senses | 3 |
| Urinary | 28 |
# CARM classification
* The number of reactions is greater than the number of reports as one report may include one or more suspected reactions.
Gastrointestinal reactions were the most common adverse events reported. These reports (excluding cases of bleeds) accounted for 42% of all reports (124/295). The majority of reactions reported were considered non-serious by CARM and may be expected based on clinical data provided in the report, in addition to being described in the data sheet.
The eleven most commonly reported suspected adverse reactions are outlined in Table 2 on page 30.
There were a total of 20 medication errors reported to CARM. These reports generally described cases where the patient was either incorrectly switched from warfarin, given the incorrect dose for their age, or should not have been treated with dabigatran at all, such as those with severe renal impairment. The majority of these reports were received within the first few weeks of funding. Since the beginning of October only two reports of medication error were received.
Of the 295 reports, 78 (26%) were considered serious by CARM, as detailed in Figure 1.
| Figure 1: Dabigatran serious reports | |||
|---|---|---|---|
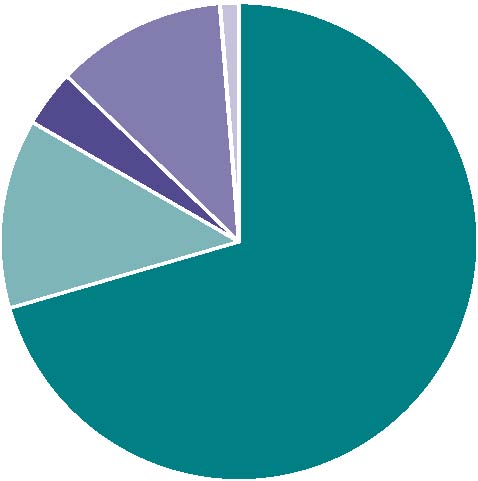 |
|
Patient hospitalised | 55 |
|
|
Patient died | 10 | |
|
|
Intervention required | 3 | |
|
|
Life threatening | 9 | |
|
|
Persisting disability | 1 | |
Table 2: Most commonly reported suspected adverse reactions to dabigatran
| Adverse event | Number | Category |
|---|---|---|
| Dyspepsia | 60 | Alimentary |
| Rectal bleeding | 47 | Alimentary |
| Diarrhoea | 26 | Alimentary |
| Melaena | 24 | Alimentary |
| Medication error | 20 | Procedure Related |
| Abdominal pain | 19 | Alimentary |
| Haematuria | 17 | Urinary |
| Headache | 14 | Nervous System |
| Gastroesophageal reflux | 13 | Alimentary |
| GI haemorrhage | 13 | Alimentary |
| Dyspnoea | 13 | Alimentary |
CARM considered that in all of the fatal cases received up to 7 November
2011, the death was unrelated to treatment with dabigatran. In these cases
the death was attributed to another medicine or unrelated event such as
pneumonia.
Adverse reaction data indicates that bleeding is the most important risk associated with dabigatran, specifically:
- Overall 42% (124/295) of the reports mentioned bleeding as a possible adverse reaction.
- Fifty-one (65%) of the 78 serious cases mentioned a bleeding event.
- Forty-one percent (51/124) of all cases mentioning bleeding as an adverse reaction were serious.
- In 29 of the serious cases (57%) the patient was also taking another medicine which can cause bleeding.
- Of all the serious cases more than half were reported in patients aged 80 years or above.
Medsafe has reviewed the incidence of adverse reactions in elderly patients. To date the data indicates there is a slightly higher incidence of reports of suspected adverse reactions in patients aged 80 years and above. Medsafe continues to closely monitor the safety of dabigatran in older patients.
When assessing the risks associated with dabigatran, an obvious medicine to compare it with is warfarin. A comparison using spontaneous adverse reaction reports is not strictly advisable due to the length of time warfarin has been available and the stimulated reporting of suspected adverse reactions to dabigatran. However, a crude comparison does provide some context for readers.
In the period between 1 Jan 2006 and 31 Dec 2010, CARM received 127 adverse reaction reports for warfarin. Sixty-five percent of these reports were considered to be serious, including 15 fatal reports. Of these fatal reports, CARM considered that warfarin had contributed to the patient’s death in 11 cases.
The global number of adverse reaction describing serious bleeds, including New Zealand data, is noted to be lower than that seen in the clinical studies published to date. Healthcare professionals are reminded that all anticoagulants have risks including the risk of bleeding, and patients taking these medicines should be closely monitored for signs of bleeding.
Please continue to report all suspected adverse reactions to any anticoagulant to CARM.





