Published: November 2009
Publications
Complementary Medicine Corner: Reporting adverse reactions
Prescriber Update 30(4): 26
November 2009
Prescribers are encouraged to ask patients about their use of complementary medicines and to report all suspected adverse reactions to complementary and alternative medicines to CARM.
Adverse reactions to complementary and alternative medicines are currently listed as an Adverse Reaction of Current Concern. This includes suspected reactions to herbal medicines, bee products, homoeopathic products, dietary supplements, minerals, and any other medicines containing animal or plant extracts.
Overview of reports to CARM
The number of reports of suspected adverse reactions to complementary medicines in New Zealand is low. From 1992 to March 2009 only 344 reports identified a complementary medicine as a suspect medicine. Of the 344 reports received 25% described serious adverse events.1
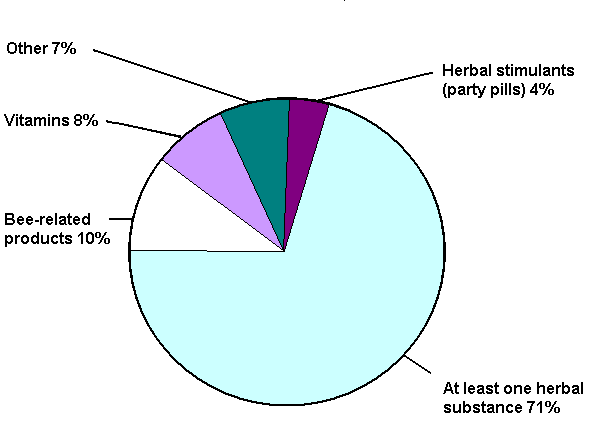
Some reports included more than one complementary medicine; a total of 403 complementary medicines were listed as suspect medicines in the reports. Grouping these products into five broad categories indicates that most suspected products contained at least one herbal substance.
The reasons why reporting is low for complementary medicines is unclear but some possible reasons include:
- Uncertainty about what can be reported.
- Lack of association between the use of complementary medicines and the adverse reaction (assumption that natural = safe).
- Lack of discussion between patients and healthcare professionals about the use of complementary medicines.
Information to include when reporting
Increasing the number of good quality reports increases CARM and Medsafe’s ability to detect potential side effects and assists in the assessment of risk. Reporting also helps in the identification of interactions with conventional medicines.
When reporting a suspected adverse reaction to a complementary or alternative medicine use the same reporting form as for medicines. It is also important to include as much information about the suspect product as possible, such as:
- The brand name (if it has one).
- The list of ingredients stated on the product label.
- Details of the manufacturer.
- Ideally, a copy of the package label.
For serious adverse reactions, healthcare professionals should consider asking the patient to retain the product in case testing is needed. Medsafe may decide to have the product tested if it is suspected to contain an undeclared ingredient such as a prescription medicine. Prescription medicines have been found in complementary medicines in New Zealand, for example in those used for slimming or erectile dysfunction.
Footnote
- Serious adverse event - events where the patient either died, required hospitalisation or prolonged existing hospitalisation, required intervention to prevent permanent disability/incapacity, resulted in a congenital abnormality, or where the event was life-threatening.





