Published: September 2012
Publications
Antibiotics and Liver Injury - Be Suspicious!
Prescriber Update 33(3): 26–27
September 2012
Prescribers are advised to be aware of the risk of liver injury associated with antibiotic treatment. Early recognition is essential as withdrawal of the causative antibiotic is the most effective treatment1. Specialist advice should be sought in all cases of severe liver injury and in patients who fail to improve despite withdrawal of the antibiotic.
Drug-induced liver injury
Drug-induced liver injury (DILI) can be classified as hepatocellular, cholestatic or mixed depending on the specific liver function test abnormalities that occur. DILI has an estimated incidence of 1 in 10,000 to 1 in 100,000. As with other liver diseases, DILI can present with jaundice, malaise, abdominal pain, unexplained nausea and anorexia. There are no specific signs, symptoms or tests that can confirm a diagnosis of DILI.
Antibiotic-associated DILI
Antibiotics are a common cause of DILI, probably because of the high rate of exposure in the community. Most cases are idiosyncratic and are therefore rare, unpredictable (from the pharmacology of the antibiotic) and largely dose-independent1, 2. The characteristics of DILI associated with specific antibiotics are summarised below (Table 1).
Table 1: Estimated frequency and characteristics of DILI associated with selected antibiotics2
| Antibiotic | Incidence and liver injury | Onset | Time to recovery |
|---|---|---|---|
| Flucloxacillin | 1.8-3.6 per 100,000 prescriptions Cholestatic |
Can be early (1-9 weeks after starting) or delayed after treatment has stopped | Usually within 12 weeks of stopping. 30% have a protracted course |
| Amoxicillin/ Clavulanic acid |
1-17 per 100,000 prescriptions Hepatocellular, cholestatic or mixed |
Within 4 weeks of starting but typically after stopping | Within 16 weeks of stopping therapy |
| Ceftriaxone | Up to 25% adults and 40% children develop cholelithiasis | After 9-11 days of treatment | Within 2-3 weeks of stopping |
| Erythromycin | < 4 cases per 100,000 prescriptions Cholestatic |
Within 10-20 days of starting | Within 8 weeks of stopping |
| (Trimethoprim/ Sulfamethoxazole) Cotrimoxazole |
< 2 per 10,000 prescriptions Cholestatic or mixed |
Unknown | Within a few weeks of stopping |
| Doxycycline | < 1 per 18 million daily doses Cholestatic |
Long latency of over 1 year | Variable. Most recover on stopping |
| Ciprofloxacin | Isolated cases only Hepatocellular and cholestatic |
Unknown | Unknown |
Risk factors
Genetic variability is considered to be the most important risk factor, although specific genetic markers have not yet been elucidated for most antibiotics1. Other potential risk factors include1:
- previous hepatotoxic reaction to a specific antibiotic
- female sex
- increasing age
- comorbid illnesses
An important exception are tetracyclines, where high doses seem to be a predictor of liver injury2.
Diagnosis and treatment
Diagnosis requires a temporal association with antibiotic use and exclusion of other causes of acute liver injury (eg, alcohol, viral hepatitis, autoimmune liver disease, metabolic liver disease, ischaemic hepatitis and extra-hepatic biliary obstruction)3. The pattern of liver injury may also aid diagnosis (Table 1).
Treatment consists primarily of withdrawal of the causative antibiotic and supportive care if required. Most cases are mild and self-limiting1. However, rare cases of acute liver failure and death have been reported1. Chronic liver disease is a very rare complication but is more likely to develop if the antibiotic is continued despite evidence of liver injury.
New Zealand case reports
The Centre for Adverse Reactions Monitoring (CARM) has received a total of 360 reports of liver injury associated with the use of non-tuberculosis antibiotics since January 2000. Most reports were in adults aged over 50 years (71%), with 13 reports in patients aged less than 20 years. Seven reports (2%) involved a fatality.
The majority of CARM reports of liver injury were associated with β-lactam penicillins (Figure 1). Amoxicillin/clavulanic acid, flucloxacillin and erythromycin were the antibiotics most often implicated in the development of liver injury in New Zealand.
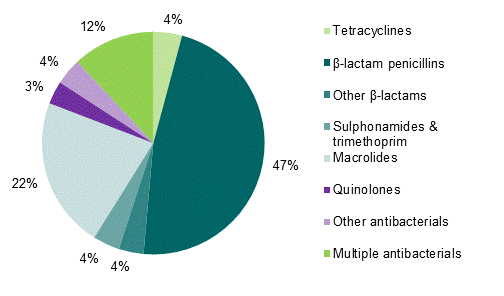
Figure 1: Classes of non-tuberculosis antibiotics associated with liver injury in New Zealand
References
- Polson JE. 2007. Hepatotoxicity due to antibiotics. Clinics in Liver Disease 11: 549-61, vi.
- Andrade RJ, Tulkens PM. 2011. Hepatic safety of antibiotics used in primary care. Journal of Antimicrobial Chemotherapy 66: 1431-46.
- Hussaini SH, Farrington EA. 2007. Idiosyncratic drug-induced liver injury: an overview. Expert Opinion on Drug Safety 6: 673-84.





