Revised: 23 May 2013
Safety Information
Medical Device Safety Issues
Management of Patients with Metal on Metal Hip Implants.
Medsafe and the New Zealand Orthopaedic Association (NZOA) (www.NZOA.org.nz) support the advice to healthcare professionals issued by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) on the management of patients implanted with metal on metal hip replacements.
Issue
The majority of patients who receive a metal on metal hip replacement do well with the implant and are thought to be at low risk of developing serious problems. A small number of patients implanted with metal on metal hip replacements may develop a metal debris problem that can lead to a progressive soft tissue reaction in proximity to the joint. The metal debris problem does not occur in every patient but in some patients it occurs through the build-up of microscopic metal debris through wear at the articular surface of the joint. The issue may be monitored through a heightened level of trace amounts of the metals cobalt and chromium in the blood of a patient. Early revision surgery of poorly performing hip replacements is likely to lead to a better outcome of revision surgery.
Action
The attached table developed by the MHRA details the management of patients with implanted metal on metal hip replacements. This management plan is supported by both Medsafe and NZOA and can be used as a set of guidelines or a protocol on how to manage patients with metal on metal implants. Where appropriate a revision of the implant should be completed as early as possible.
The types of replacement are divided into four groups:
- MoM hip resurfacing implants
- MoM total hip replacements with head diameter <36mm
- MoM total hip replacements with head diameter ≥36mm
- DePuy ASR™ hip replacements comprising:
- - ASR™ acetabular cups for hip resurfacing arthroplasty or total hip replacement
- - ASR™ surface replacement heads for hip resurfacing arthroplasty
- - ASR™ XL femoral heads for total hip replacement.
The table has recommendations for patients who are symptomatic and also those who are asymptomatic.
Importantly all patients with implanted with a metal on metal implant that has a large diameter head (36 mm or greater) will require annual follow-up for the life of the implant.
Medsafe and the NZOA are providing this advice to you so that healthcare professionals can provide consistent advice to patients that seek consultations regarding their implanted hip replacement.
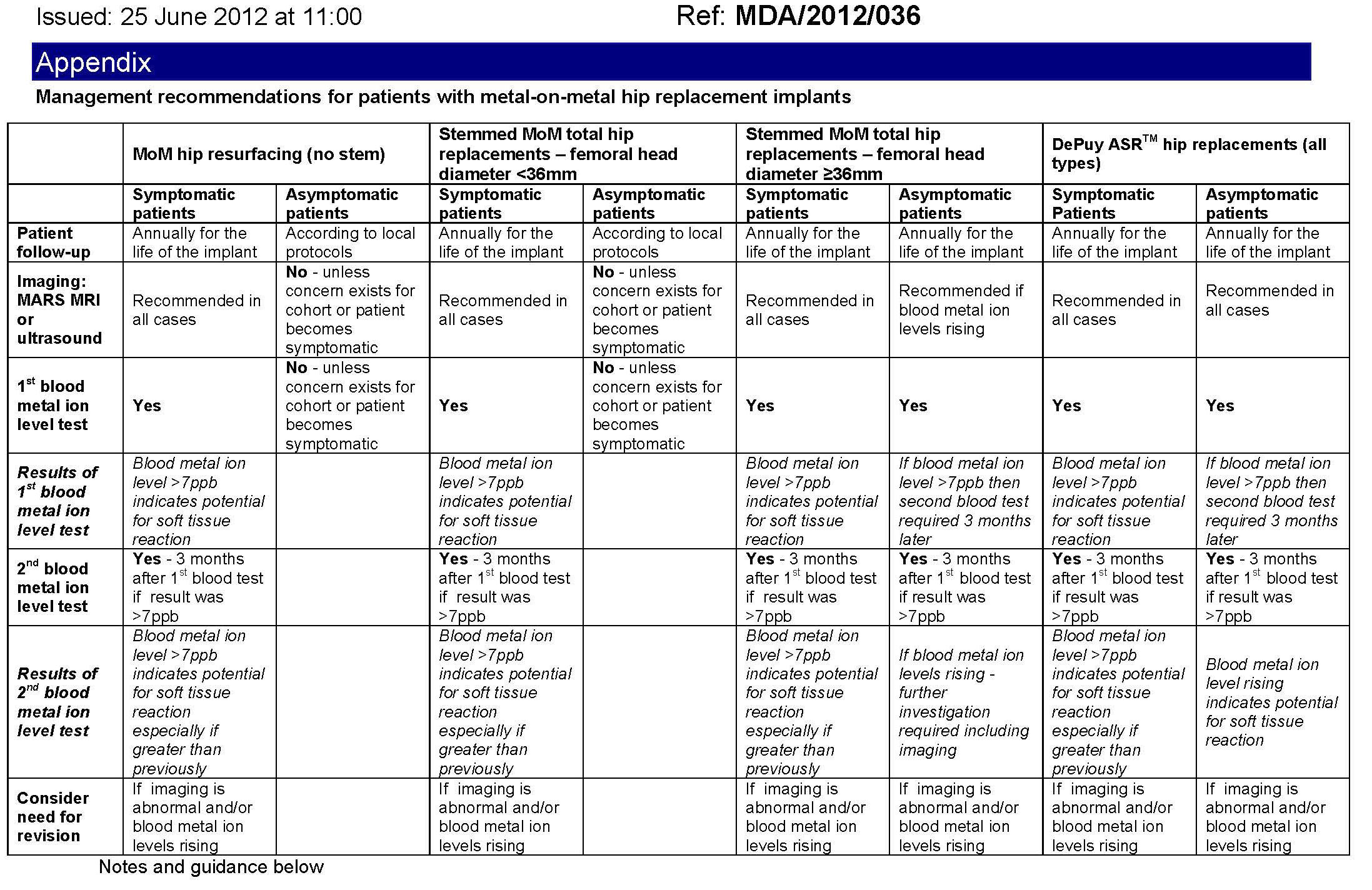
Table was updated on 27 August 2012
Table footnotes:
- Blood metal ion testing to be in whole blood
- 7 parts per billion (ppb) equals 119 nmol/L cobalt or 134.5 nmol/L chromium
Guidance notes
- On the basis of current knowledge, this chart has been produced as a guide to the management of these patients. It will not necessarily
- cover all clinical situations and each patient must be judged individually.
- MARS MRI scans (or ultrasound scans) should carry more weight in decision making than blood ion levels alone.
- Patients with muscle or bone damage on MARS MRI are those of most concern. A fluid collection alone around the joint in an asymptomatic patient, unless it is very large can be safely observed with interval scanning.
- Local symptoms include pain and limping.





