Published: 2 June 2022
Publications
Targeting SARS-CoV-2: An overview of COVID-19 treatments
Published 2 June 2022
Prescriber Update 43(2): 14–17
June 2022
The number of COVID-19 treatments approved for use in New Zealand is increasing. Here we provide a summary of when they are used, how they work, and list examples from each drug class.
COVID-19 treatments in the different stages of infection
Early in COVID-19 infection, viral replication is thought to drive many of the initial signs and symptoms. Therefore, neutralising antibodies that prevent the virus from attaching or entering the cell and small molecule direct-acting antivirals that inhibit viral replication are anticipated to have the greatest effect on limiting disease severity (Figure 1).1
In the later course of infection, immune modulators are likely to be beneficial, particularly if disease is driven by immune dysregulation leading to excessive inflammation (Figure 1).1,2
Figure 1: SARS-CoV-2 virus and human host targets and potential COVID-19 treatments
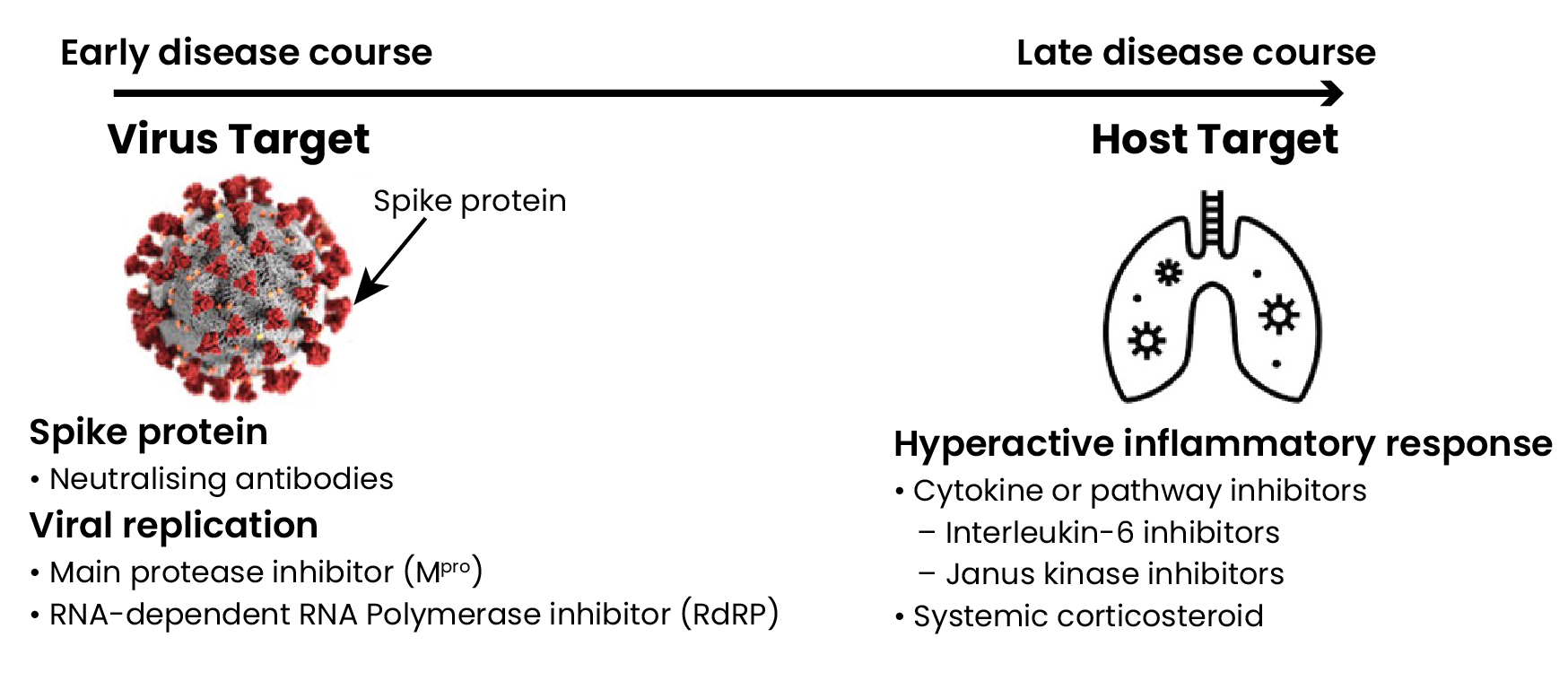
Neutralising antibody treatments targeting the SARS-CoV-2 spike protein
The spike (S) protein on the surface of SARS-CoV-2 mediates attachment and entry of the virus to the host cell. Targeting this interaction with neutralising antibodies, such as monoclonal antibodies, can interfere with the virus entering the host cell.3
As the virus replicates, spontaneous mutations arise in the viral code. These mutations become selected, known as selective pressure, when they confer a survival advantage in the presence of the drug.4 The S protein is under heavy selective pressure, which has led to variant mutations occurring within the S protein.5 For example, the highly transmissible Omicron variant has up to 32 mutations in the S protein. Neutralising antibodies may have reduced activity against different variants of SARS-CoV-2. The susceptibility of circulating variants must be considered when prescribing a neutralising antibody treatment.6
Some medicines combine two types of neutralising monoclonal antibodies to reduce the risk of resistance associated with monotherapy.7
Small molecule direct-acting antiviral treatments
Small molecule direct-acting antivirals target critical stages of the viral replication cycle to suppress or inhibit viral replication.7 Some targets for this class include the viral main protease (Mpro) and RNA-dependent RNA polymerase (RdRp).
Mpro inhibitors
The SARS-CoV-2 virus Mpro, also known as 3C-like protease (3CLpro), performs the first major step of viral replication.3 It activates the proteins needed to form the viral replication complex.3 Therefore, inhibiting Mpro prevents the virus from replicating.
The SARS-CoV-2 virus Mpro structure is different from that of human proteases, making it a highly specific target for therapeutics and reducing the risk of severe side effects.3 Nirmatrelvir (with ritonavir) is a SARS-CoV-2 Mpro inhibitor.
Nucleoside analogues
RdRp is an enzyme responsible for replicating the SARS-CoV-2 RNA genome. After replication, the RdRp translates and transcribes the RNA to structural and accessory proteins.3
RdRp incorporates nucleoside analogues into the viral RNA strand. Remdesivir causes chain termination, which stops RNA synthesis. Molnupiravir causes mutations to accumulate over cycles, leading to viral error catastrophe (ie, where there are so many mutations that the virus is no longer viable).7
Resistance
Mpro and RdRp are highly conserved across coronaviruses and have a high barrier to resistance, as significant mutations in these enzymes would likely reduce pathogen virulence.3 However, there is little published evidence on the potential for SARS-CoV-2 to develop resistance to therapies that target these enzymes. In other viral infections, resistance to nucleoside analogue monotherapies emerges extremely readily, whereas the inhibitory activity of protease inhibitors is more durable.7
Immune modulators
Immune modulators have an important role during the later course of infection, if a hyperactive inflammatory response occurs.1 Interleukin-1 and interleukin-6 are likely the most relevant pro-inflammatory cytokines involved.8 The choice of modulator ranges from non-specific and broad, such as corticosteroids, to very targeted, such as inhibiting one specific cytokine.8
Summary
Table 1 provides examples of COVID-19 medicines from each drug class. Note that some medicines may not be approved for use in New Zealand. The Medsafe website has the approval status of COVID-19 treatment applications.
Refer to the medicine data sheet for prescribing and adverse event information for approved medicines.
Table 1: Examples of COVID-19 medicines from each drug class
| Drug class | Medicinea | Notesb |
|---|---|---|
| Neutralising antibodies targeting S protein | Casirivimab + imdevimab | See the Ronapreve
data sheet.
|
| Tixagevimab + cilgavimab | Two long-acting monoclonal antibodies that bind to non-overlapping areas of the S protein.c | |
| Sotrovimab | Single monoclonal antibodies that bind to the S protein.d | |
| Bebtelovimab | ||
| Mpro inhibitors (Small molecule direct-acting antivirals) | Nirmatrelvir + ritonavir | See the Paxlovid
data sheet.
|
| Nucleoside analogues (Small molecule direct-acting antivirals) | Molnupiravir | See the
Lagevrio data sheet.
|
| Remdesivir | Undergoing
assessment. Proposed to cause RNA chain termination. See the New Zealand Formulary remdesivir drug monograph. |
|
| Immune modulators | Tocilizumab | See the Actemra
data sheet.
|
| Baricitinib | A Janus kinase (JAK) inhibitor that may also have antiviral
properties.e See the New Zealand Formulary baricitinib drug monograph. |
|
| Dexamethasone | See the Dexmethsone
data sheet.
|
Notes:
- Examples given may not have approval for use in New Zealand. Information current to 12 May 2022. Emerging variants of concern and the efficacy of certain medicines may reduce. Refer to national or local guidelines for the latest information.
- Unless otherwise referenced, the medicine information is sourced from the respective New Zealand data sheet.
- AstraZeneca UK Limited. 2022. Summary of Product Characteristics for Evusheld 17 March 2022. URL: gov.uk/government/publications/regulatory-approval-of-evusheld-tixagevimabcilgavimab/summary-of-product-characteristics-for-evusheld (accessed 14 April 2022).
- National Institutes of Health. 2022. NIH COVID-19 Treatment Guidelines: Therapeutic Management of Nonhospitalized Adults With COVID-19. 8 April 2022. URL: covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/ (accessed 14 April 2022).
- Kim AY and Gandhi RT. 2022. COVID-19: Management in hospitalized adults. In: UpToDate 24 January 2022. URL: uptodate.com/contents/covid-19-management-in-hospitalized-adults (accessed 13 April 2022).
More information
- New Zealand Formulary: COVID-19 treatments
- Ministry of Health: COVID-19: Advice for all health professionals
- Pharmac: COVID-19 treatments portfolio and Access criteria
References
- Gandhi RT. 2021. The multidimensional challenge of treating coronavirus disease 2019 (COVID-19): Remdesivir Is a foot in the door. Clinical Infectious Diseases 73(11): e4175–8. DOI: 10.1093/cid/ciaa1132 (accessed 17 April 2022).
- Ragab D, Haitham SE, Mohamed T, et al. 2020. The COVID-19 cytokine storm; What we know so far. Frontiers in Immunology 16(11): 1446. DOI: 10.3389/fimmu.2020.01446 (accessed 27 April 2022).
- Krumm ZA, Lloyd GM, Francis CP, et al. 2021. Precision therapeutic targets for COVID-19. Virology Journal 18(1): 66. DOI: doi.org/10.1186/s12985-021-01526-y (accessed 13 April 2022).
- World Health Organization. 2022. Therapeutics and COVID-19: Living Guideline 22 April 2022. URL: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3 (accessed 29 April 2022).
- Rosales R, McGovern L, Rodriguez ML, et al. 2022. Nirmatrelvir, molnupiravir, and remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. bioRxiv preprint 19 January 2022. DOI: doi.org/10.1101/2022.01.17.476685 (accessed 19 April 2022).
- Cohen P and Gebo K. 2022. COVID-19: Outpatient evaluation and management of acute illness in adults. In: UpToDate 15 April 2022. URL: uptodate.com/contents/covid-19-outpatient-evaluation-and-management-of-acute-illness-in-adults (accessed 19 April 2022).
- New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG). 2021. NERVTAG:Antiviral drug resistance and the use of directly acting antiviral drugs (DAAs) for COVID-19, 8 December 2021 8 December 2021. URL: gov.uk/government/publications/nervtag-antiviral-drug-resistance-and-the-use-of-directly-acting-antiviral-drugs-daas-for-covid-19-8-december-2021/nervtag-antiviral-drug-resistance-and-the-use-of-directly-acting-antiviral-drugs-daas-for-covid-19-8-december-2021 (accessed 13 April 2022).
- van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. 2022. A guide to immunotherapy for COVID-19. Nature Medicine 28(1): 39-50. DOI: doi.org/10.1038/s41591-021-01643-9 (accessed 19 April 2022).





