Published: 1 September 2016
Publications
Drug-induced Gynaecomastia
Prescriber Update 37(3): 37
September 2016
A recent report to the Centre for Adverse Reactions Monitoring (CARM) described
a male patient who developed bilateral gynaecomastia after taking omeprazole
(20 mg twice a day) for 10 years1.
Gynaecomastia is a benign proliferation of male breast glandular tissue caused by an imbalance between the actions of oestrogens relative to the actions of androgens on breast tissue2-6.
Medicines have been estimated to cause 10% to 25% of all cases of gynaecomastia2,4,5.
Diagnosis
In male patients proliferation of breast tissue may be due to gynaecomastia, pseudo-gynaecomastia or breast cancer. A physical examination is necessary to distinguish the cause2-5. Breast imaging and ultrasonography may be considered if breast cancer is suspected2,3,5.
Once gynaecomastia is confirmed, consider reversible causes including medicines and over-the-counter products (see Causal Medicines)2-6.
In patients with no apparent cause of gynaecomastia, laboratory tests may be useful. Tests may include liver, renal and thyroid function2-5. A limited hormonal work-up (eg, testosterone, oestradiol, LH, FSH, hCG, and prolactin) may be useful to detect hormonal imbalances2-5.
Causal Medicines
Medicines may cause gynaecomastia through a number of mechanisms including:4,5
- increased serum oestrogen levels or oestrogen-like activity
- decreased testosterone levels
- hypogonadism
- anti-androgenic effects
- increased prolactin levels.
Many commonly prescribed medicines have been reported to cause gynaecomastia5. Table 1 provides examples of medicines that have been associated with gynaecomastia2,3.
Table 1: Medicines reported to be associated with gynaecomastia [this is not an exhaustive list]2-7
| Quality of Evidence* | Examples of Medicines and/or Medicine Classes [in alphabetical order] |
|---|---|
| Good | Bicalutamide, cyproterone, dutasteride, finasteride, flutamide, goserelin, ketoconazole, leuprorelin, oestrogens, spironolactone |
| Fair | Alkylating agents, anabolic steroids, efavirenz, nifedipine, omeprazole, opioids, risperidone, verapamil |
*Classification of quality of evidence for strength of medicine associations with gynaecomastia. Good: Systematic review of randomised controlled trials, randomised placebo controlled trial, or prospective cohort studies with or without concurrent controls plus good pathophysiological explanation. Fair: Retrospective studies, case-control studies or case series with good pathophysiological explanation.
Other reported non-medicine causes of gynaecomastia include alcohol, amphetamines, heroin, marijuana (cannabis) and phytoestrogens (in some herbal medicines)2,3,4,7.
New Zealand Cases
Up to 30 June 2016, 124 reports of gynaecomastia suspected to be caused by medicines have been reported to CARM (4.8% of reports were in women). These reports involved 135 suspected medicines as more than one medicine was described in some reports. The average age in these reports was 58 years.
The 10 most frequently reported medicines are summarised in Figure 1. These medicines comprise 65% of total reports. Of these (n=81), time to onset was reported in 67 reports. In six reports, the onset was reported to have occurred within one month and in 61 reports, the onset was reported to be one year or longer.
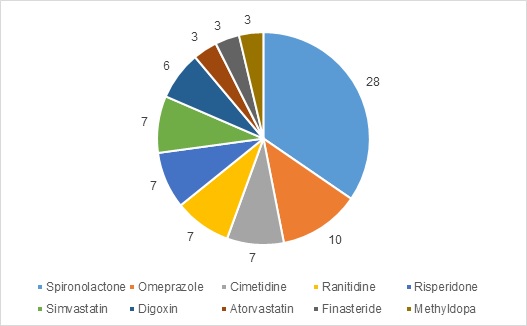
Figure 1: Top 10 medicines associated with gynaecomastia in the CARM database (number of reports)
Management
If medicine-induced gynaecomastia is suspected, the medicine should be withdrawn or discontinued if possible2,4. Switching to a medicine of the same class, but with a lower association with gynaecomastia may prevent re-emergence of gynaecomastia2.
Within one month of discontinuing the causal medicine, a reduction in tenderness and softening of the glandular breast tissue should occur. However, if the gynaecomastia has been present for more than one year, the presence of fibrosis may mean that surgery is required2-6.
Please continue to report any adverse reactions to CARM. Reports can be submitted on paper or electronically (https://nzphvc.otago.ac.nz/).
References
- CARM case ID 119664. URL: www.medsafe.govt.nz/Projects/B1/adrsearch.asp
- Deepinfer F, Braunstein GD. 2012. Drug-induced gynecomastia: an evidence-based review. Expert Opinion on Drug Safety 11(5): 779-795.
- Braunstein GD. 2007. Gynecomastia. The New England Journal of Medicine 357: 1229-1237.
- Eckman A, Dobs A. 2008. Drug-induced gynecomastia. Expert Opinion on Drug Safety 7(6): 691-702.
- Narula HS, Carlson HE. 2014. Gynaecomastia – pathophysiology, diagnosis and treatment. Nature Reviews Endocrinology 10: 684-698.
- Carlson HE. 2011. Approach to the Patient with Gynecomastia. The Journal of Clinical Endocrinology & Metabolism 96: 15-21.
- Bowman JD, Kim H, Bustamante JJ. 2012. Drug-Induced Gynecomastia. Pharmacotherapy 32(12): 1123-1140.





